Meng-Yue Wang, Xue-Qing Zhu, Xing-Long Zhang, Rui-Li Guo, Qiong Jia and Yong-Qiang Wang
Org. Biomol. Chem., 2020,18, 5918-5926
DOI:
10.1039/D0OB01160F,
Paper
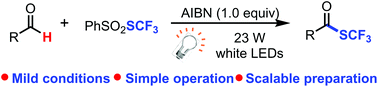
We report herein an efficient, economical, and scalable trifluoromethylthiolation of aldehydes to generate trifluoromethylthioesters via a visible light-promoted radical process. The transformation features cheap reagents, simple operation, a broad substrate scope, and especially no metal involved in the reaction. Trifluoromethylthiolations of several complex aldehyde-containing bioactive compounds have been realized; thus the approach has the potential to be an important tool for the late-stage functionalization of advanced synthetic intermediates and bioactive molecules, and should have many applications in medicinal chemistry.