Jiajie Deng, Xueting Wu, Guiling Guo, Xiaohu Zhao and Zhipeng Yu
Org. Biomol. Chem., 2020,18, 5602-5607
DOI:
10.1039/D0OB01027H,
Paper
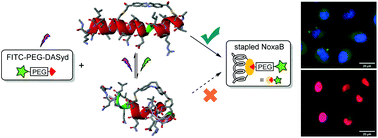
A photo-induced 1,3-dipolar cycloaddition between nitrile imine and highly ring-strained N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond as a dipolarophile was discovered. The photo-isomerization of carbon-bridged octocyclic azobenzene (CBOA) into its trans-configuration accelerates the ligation reaction at a very rapid rate (28 400 M−1 s−1). The CBOA-based photo-click reaction was proved to be bioorthogonal. In addition, the NoxaB peptide was successfully cross-linked by a CBOA stapler which plays a dual role: photo-control of the conformation of the peptide and photo-conjugation of probes in live cells.
N double bond as a dipolarophile was discovered. The photo-isomerization of carbon-bridged octocyclic azobenzene (CBOA) into its trans-configuration accelerates the ligation reaction at a very rapid rate (28 400 M−1 s−1). The CBOA-based photo-click reaction was proved to be bioorthogonal. In addition, the NoxaB peptide was successfully cross-linked by a CBOA stapler which plays a dual role: photo-control of the conformation of the peptide and photo-conjugation of probes in live cells.