Synthesis of 2-aryl quinazolinones via iron-catalyzed cross-dehydrogenative coupling (CDC) between N–H and C–H bonds†
Abstract
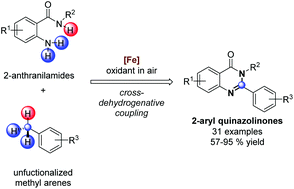
Herein, we describe the direct synthesis of quinazolinones via cross-dehydrogenative coupling between methyl arenes and anthranilamides. The C–H functionalization of the benzylic sp3 carbon is achieved by di-t-butyl peroxide under air, and the subsequent amination–aerobic oxidation process completes the annulation process. Iron catalyzed the whole reaction process and various kinds of functional groups were tolerated under the reaction conditions, providing 31 examples of 2-aryl quinazolinones using methyl arene derivatives in yields of 57–95%. The synthetic potential has been demonstrated by the additional synthesis of aryl-containing heterocycles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...