Palladium catalyzed asymmetric allylic alkylation of isoquinolinedione derivatives in the preparation of quaternary carbon stereocenters†
Abstract
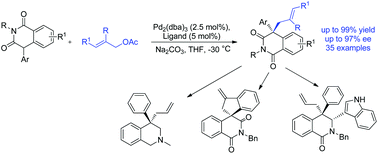
A highly enantioselective allylic alkylation of isoquinolinedione derivatives under palladium catalysis was developed in the preparation of quaternary carbon stereocenters. Under standard reaction conditions, excellent yields and enantioselectivities were realized and the products could be transformed into dihydroisoquinolone with vicinal chiral carbon centers or THIQ core structures in short steps with high yields.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...