Biselyngbyolides A & C: their total synthesis and anticancer activities†
Abstract
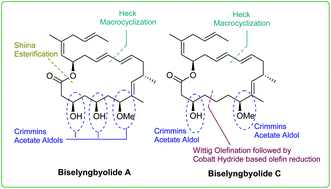
Convergent strategies for the first total synthesis of biselyngbyolide C and an alternative route for the total synthesis of biselyngbyolide A have been developed. The key strategic feature in this study is Heck macrocyclization. The use of intramolecular Heck coupling for biselyngbyolide B was demonstrated by us earlier; however such a strategy has not been explored further for the other members of this family of natural products, in particular, where sensitive skipped olefins are involved. The other highlights of this synthetic study include iterative Crimmins acetate aldol and Wittig olefination processes, followed by the less explored cobalt-hydride-based reduction of an activated olefin and Shiina esterification. Our synthetic study enabled us to amend the reported NMR data of biselyngbyolides A and C. An evaluation of the anticancer activities of both biselyngbyolides A and C revealed that the apoptosis generated in cancer cells followed an intrinsic pathway.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...