Open Access Article
Open Access ArticleSynthesis of unsymmetrical benzils via palladium-catalysed α-arylation–oxidation of 2-hydroxyacetophenones with aryl bromides†
Takanori
Matsuda
 * and
Souta
Oyama
* and
Souta
Oyama
Department of Applied Chemistry, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: mtd@rs.tus.ac.jp
First published on 23rd April 2020
Abstract
A diverse set of unsymmetrically substituted benzils were facilely synthesised by a cross-coupling reaction between 2-hydroxyacetophenones and aryl bromides in the presence of a palladium catalyst. Experimental studies suggested a reaction mechanism involving a one-pot tandem palladium-catalysed α-arylation and oxidation, where aryl bromides play a dual role as mild oxidants as well as arylating agents.
Introduction
1,2-Diketones are members of an important class of molecules with diverse application potential in various fields. They are useful building blocks for the synthesis of a range of carbo- and heterocyclic compounds,1 and 1,2-diketone-derived compounds have been utilised as ligands for transition metals.2 Among these 1,2-diketones, benzils (diphenylethanediones) are recognised as privileged scaffolds that have distinct properties, and they can be converted into compounds with vicinal diphenyl groups. Benzils are generally synthesised via oxidation of the corresponding benzoins,3 diarylacetylenes,4 or other 1,2-diphenyl derivatives.5 Thus, the traditional synthesis of unsymmetrical benzils necessitates the preparation of unsymmetrical starting materials, which can complicate the process considerably. In this context, significant advances have recently been made, whereby coupling strategies offer a viable and effective procedure for the synthesis of unsymmetrical benzils.6,7 In particular, a reaction employing an aryl halide as the aryl source would be advantageous, as a large number of aryl halides are currently commercially available and relatively inexpensive.Palladium-catalysed α-arylation of ketones with aryl halides, enabling cross-coupling between an electrophilic aryl group and a nucleophilic ketone enolate, represents a versatile and robust method for the synthesis of α-aryl ketones.8 Although the α-arylation of other carbonyl compounds, such as esters and aldehydes, as well as nitriles, and nitroalkanes has been well established,9 to date, there have been no reports on a reaction utilizing α-hydroxy ketones as nucleophiles. In this paper, we report that palladium(0)-catalysed α-arylation of 2-hydroxyacetophenones with aryl bromides produces benzoins, which are subsequently oxidised to benzils through the action of aryl bromides as mild oxidants, under catalytic conditions. In reactions of α-hydroxy ketones with two nucleophilic sites, C-arylation is particularly favoured over O-arylation.10 Moreover, a control experiment revealed that 2-hydroxyacetophenones are more prone to α-arylation than acetophenone.
Results and discussion
2-Hydroxyacetophenone (1a) and 4-bromotoluene (2a, 2 equiv.) were heated in toluene at 100 °C in the presence of 10 mol% Pd(PPh3)4 as the catalyst and NaOt-Bu as a base for 24 h (Table 1, entry 1). The reaction primarily resulted in a reductive homocoupling to afford a biaryl, and no cross-coupling was observed. In contrast, when [PdCl(allyl)]2 was employed as the catalyst, cross-coupling between 1a and 2a occurred, but the benzil product 3aa was isolated in only 9% yield (entry 2). The anticipated α-arylation product, benzoin, was not detected in the reaction mixture, indicating that oxidation had occurred concomitantly during the reaction. To increase the product yield, we examined various phosphine ligands and found that XPhos11 was the most effective (36% yield) among those examined (entries 3–8). As for the palladium complexes, [PdCl(allyl)]2 was found to be the complex of choice for this reaction (entries 8–11).| Entry | Pd catalyst (mol%) | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.100 mmol), 2a (0.200 mmol), Pd complex (10 mol%), phosphine ligand (20/10 mol% for monodentate/bidentate), NaOt-Bu (0.200 mmol), and toluene (0.5 mL) at 100 °C for 24 h. b Isolated yield. | ||
| 1 | Pd(PPh3)4 (10) | <5 |
| 2 | [PdCl(allyl)]2 (5) | 9 |
| 3 | [PdCl(allyl)]2/PPh3 (5/20) | <5 |
| 4 | [PdCl(allyl)]2/DPPE (5/10) | 14 |
| 5 | [PdCl(allyl)]2/XANTPHOS (5/10) | 5 |
| 6 | [PdCl(allyl)]2/DavePhos (5/20) | 20 |
| 7 | [PdCl(allyl)]2/JohnPhos (5/10) | 35 |
| 8 | [PdCl(allyl)]2/XPhos (5/20) | 36 |
| 9 | Pd(OAc)2/XPhos (10/20) | 21 |
| 10 | Pd(OCOCF3)2/XPhos (10/20) | 31 |
| 11 | Pd2(dba)3/XPhos (5/20) | 33 |
As the decomposition of benzil 3aa was observed with longer reaction times under the conditions employing NaOt-Bu, further optimisation was performed (Table 2). An extensive investigation into the choice of base (entries 1–6) indicated that the use of K3PO4 resulted in a cleaner reaction, furnishing 3aa in 62% yield within 6 h;12 the yield was further improved to 80% when the reaction time was 18 h (entry 7). Notably, the reaction proceeded smoothly without the need for stronger bases, such as alkoxides. Solvent screening confirmed that t-BuOH was the optimal solvent, giving superior results in terms of the prevention of decomposition of α-hydroxy ketone 1a (entries 7–13). Finally, the use of 2.5 equiv. of both the aryl bromide 2a and the base afforded 3aa in 90% yield (entry 14). The reaction with 5 mol% catalyst loading provided a comparable yield, while the yield deteriorated with further catalyst loading reduction to 2 mol% (entries 15 and 16). The coupling was successfully performed on a gram scale to furnish 1.2 g of 3aa in 91% yield (entry 17).
| Entry | Base | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.100 mmol), 2a (0.200 mmol), [PdCl(allyl)]2 (0.005 mmol, 10 mol% Pd), XPhos (0.020 mmol, 20 mol%), base (0.200 mmol) and solvent (0.5 mL) at 100 °C for the indicated time unless otherwise noted. b Isolated yield. c 2.5 equiv. (0.250 mmol) each of 2a and K3PO4 were used. d 2.5 mol% [PdCl(allyl)]2 (5.0 mol% Pd) was used. e 1.0 mol% [PdCl(allyl)]2 (2.0 mol% Pd) was used. f 1a (6.00 mmol) was reacted under reflux. | ||||
| 1 | NaOt-Bu | Toluene | 6 | 56 |
| 2 | KOt-Bu | Toluene | 6 | 22 |
| 3 | NaN(SiMe3)2 | Toluene | 6 | 32 |
| 4 | Cs2CO3 | Toluene | 6 | 33 |
| 5 | K2CO3 | Toluene | 6 | 35 |
| 6 | K3PO4 | Toluene | 6 | 62 |
| 7 | K3PO4 | Toluene | 18 | 80 |
| 8 | K3PO4 | 1,4-Dioxane | 18 | 72 |
| 9 | K3PO4 | THF | 18 | 70 |
| 10 | K3PO4 | ClCH2CH2Cl | 18 | 33 |
| 11 | K3PO4 | DMF | 18 | 25 |
| 12 | K3PO4 | EtOH | 18 | 33 |
| 13 | K3PO4 | t-BuOH | 18 | 80 |
| 14c | K3PO4 | t-BuOH | 18 | 90 |
| 15c,d | K3PO4 | t-BuOH | 18 | 83 |
| 16c,e | K3PO4 | t-BuOH | 18 | 25 |
| 17c,f | K3PO4 | t-BuOH | 18 | 91 |
The reaction proved efficient using an aryl triflate, providing 3aa in 98% yield (Scheme 1). A slight decrease in yield was observed for aryl chlorides. In the case of an aryl iodide, however, the yield was reduced to 52% due to the competing biaryl homocoupling.
With the optimised conditions in hand, we investigated the scope of the coupling reaction and found that a diverse array of unsymmetrical benzils, as well as the parent benzil could be effectively synthesised (Table 3). Coupling of 1a with bromobenzenes 2c–g bearing electron-donating and electron-withdrawing substituents at the meta- or para-positions afforded the corresponding benzils 3ac–ag in 61–98% yields (entries 2–6). Moreover, it was established that the reaction was effective in the presence of heteroatom substituents (entries 7–10). The reaction using 2- and 1-naphthyl bromides 2l and 2m delivered 1,2-diketones 3al and 3am in 79% and 58% yields, respectively, (entries 11 and 12). However, the yields of 3 declined considerably when ortho-substituted bromobenzenes 2n and 2o were used (entries 13 and 14). The attempted reaction with 4-bromophenol afforded only a trace amount of the desired product, and the formation of complex product mixtures was observed with 1-bromo-3-nitrobenzene and 3′-bromoacetophenone.13 Furthermore, a variety of α-hydroxy ketones 1b–k, including naphthyl and heteroaryl ketones, also underwent the coupling reaction with 2a to deliver 3ba–ka (entries 15–24).14 Finally, it was demonstrated that additional unsymmetrical benzils, including highly electronically biased 3ee, could be obtained by the coupling protocol (entries 25–31).
| Entry | 1 (Ar1) | 2 (Ar2) | 3 | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.200 mmol), 2 (0.500 mmol), [PdCl(allyl)]2 (0.010 mmol, 10 mol% Pd), XPhos (0.040 mmol, 20 mol%), K3PO4 (0.500 mmol) and t-BuOH (0.5 mL) at 100 °C for 18 h. b Isolated yield. c B(dan) = naphtho[1,8-de][1,3,2]diazaborinin-2-yl. | ||||
| 1 | 1a (Ph) | 2b (Ph) | 3ab | 88 |
| 2 | 1a (Ph) | 2c (4-t-BuC6H4) | 3ac | 86 |
| 3 | 1a (Ph) | 2d (3-MeC6H4) | 3ad | 98 |
| 4 | 1a (Ph) | 2e (4-MeOC6H4) | 3ae | 78 |
| 5 | 1a (Ph) | 2f (4-F3CC6H4) | 3af | 70 |
| 6 | 1a (Ph) | 2g (3-MeO2CC6H4) | 3ag | 61 |
| 7 | 1a (Ph) | 2h (4-FC6H4) | 3ah | 89 |
| 8 | 1a (Ph) | 2i (4-Me3SiC6H4) | 3ai | 98 |
| 9 | 1a (Ph) | 2j (3-MeSC6H4) | 3aj | 76 |
| 10 | 1a (Ph) | 2k (3-(dan)BC6H4)c | 3ak | 49 |
| 11 | 1a (Ph) | 2l (2-naphthyl) | 3al | 79 |
| 12 | 1a (Ph) | 2m (1-naphthyl) | 3am | 58 |
| 13 | 1a (Ph) | 2n (2-MeC6H4) | 3an | 45 |
| 14 | 1a (Ph) | 2o (2-MeOC6H4) | 3ao | 44 |
| 15 | 1b (4-MeC6H4) | 2a (4-MeC6H4) | 3ba | 92 |
| 16 | 1c (3-MeC6H4) | 2a (4-MeC6H4) | 3ca | 95 |
| 17 | 1d (3-MeOC6H4) | 2a (4-MeC6H4) | 3da | 78 |
| 18 | 1e (4-F3CC6H4) | 2a (4-MeC6H4) | 3ea | 64 |
| 19 | 1f (4-FC6H4) | 2a (4-MeC6H4) | 3fa | 79 |
| 20 | 1g (2-MeC6H4) | 2a (4-MeC6H4) | 3ga | 71 |
| 21 | 1h (2-naphthyl) | 2a (4-MeC6H4) | 3ha | 74 |
| 22 | 1i (1-naphthyl) | 2a (4-MeC6H4) | 3ia | 72 |
| 23 | 1j (2-furyl) | 2a (4-MeC6H4) | 3ja | 55 |
| 24 | 1k (2-thienyl) | 2a (4-MeC6H4) | 3ka | 76 |
| 25 | 1d (3-MeOC6H4) | 2e (4-MeOC6H4) | 3de | 71 |
| 26 | 1e (4-F3CC6H4) | 2e (4-MeOC6H4) | 3ee | 59 |
| 27 | 1g (2-MeC6H4) | 2f (4-F3CC6H4) | 3gf | 65 |
| 28 | 1g (2-MeC6H4) | 2l (2-naphthyl) | 3gl | 70 |
| 29 | 1h (2-naphthyl) | 2f (4-F3CC6H4) | 3hf | 62 |
| 30 | 1i (1-naphthyl) | 2f (4-F3CC6H4) | 3if | 61 |
| 31 | 1k (2-thienyl) | 2l (2-naphthyl) | 3kl | 82 |
Several control experiments were conducted to elucidate the mechanistic aspects of the coupling reaction (Scheme 2). When the reaction was terminated after 1 h, benzoin 4aa was isolated in 49% yield in addition to benzil 3aa (37%), suggesting that 4aa is the initial product, and that 3aa is subsequently formed by the oxidation of 4aa (Scheme 2A). The preference of α-arylation of α-hydroxy ketone 1a over acetophenone (5) was confirmed by a competition experiment with equimolar amounts of 1a and 5 under the standard conditions. After 1 h, 3aa and 4aa were isolated in 85% combined yield while 5, lacking the hydroxyl group, remained intact (Scheme 2B). Palladium-catalysed oxidation of alcohols using aryl halides as oxidants has been reported.15 Indeed, oxidation of benzoin (4ab) with 2a in the presence of the Pd–XPhos catalyst and K3PO4 in t-BuOH furnished benzil (3ab) in a high yield, whereas the oxidation of 4ab failed to occur in the absence of 2a (Scheme 2C). In the case of the reaction with 1-bromo-4-(tert-butyl)benzene (2c), the formation of tert-butylbenzene (42%) was detected by GC along with the quantitative formation of benzil 3ac (Scheme 2D). Another possible scenario involving an initial oxidation of an α-hydroxy ketone to a glyoxal, which is subsequently arylated, was excluded, and no arylation of phenylglyoxal (6) was observed under our conditions (Scheme 2E).16
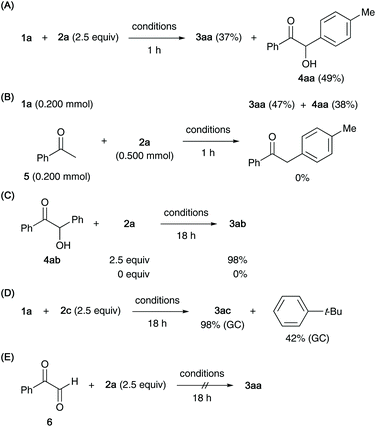 | ||
| Scheme 2 Control experiments (conditions: 5 mol% [PdCl(allyl)]2, 20 mol% XPhos, 2.5 equiv. K3PO4, t-BuOH, 100 °C). | ||
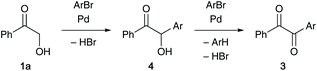
Based on these experimental observations, we conclude that the present reaction involves an initial α-arylation of 2-hydroxyacetophenone (1a) followed by oxidation of the resulting benzoin 4 to benzil 3, both of which are catalysed by a palladium complex equipped with XPhos; two equivalents of aryl bromide are consumed during the process (Scheme 3).17
Conclusions
In summary, we have developed a novel synthetic method for accessing unsymmetrical benzils starting from 2-hydroxyacetophenones, achieved by a tandem α-arylation–oxidation sequence, both of which are catalysed by a palladium–XPhos system. Readily available aryl bromides initially function as arylating agents to form C–C bonds, and then subsequently oxidise the resulting benzoins to benzils, with concomitant hydrodebromination. The widely applicable transformation can be conducted under mild and virtually redox-neutral conditions.18Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) C. Rogers, W. S. Perkins, G. Veber, T. E. Williams, R. R. Cloke and F. R. Fischer, J. Am. Chem. Soc., 2017, 139, 4052–4061 CrossRef CAS PubMed; (b) S. Sundar and R. Rengan, Org. Biomol. Chem., 2019, 17, 1402–1409 RSC; (c) A. Y. Dubovtsev, D. V. Dar'in, M. Krasavin and V. Y. Kukushkin, Eur. J. Org. Chem., 2019, 1856–1864 CrossRef CAS; (d) Y. Nakagawa, R. Sekiguchi, J. Kawakami and S. Ito, Org. Biomol. Chem., 2019, 17, 6843–6853 RSC; (e) A. Rashidizadeh, H. Ghafuri, H. R. E. Zand and N. Goodarzi, ACS Omega, 2019, 4, 12544–12554 CrossRef CAS PubMed; (f) G. Li, Y. Han, Y. Zou, J. J. C. Lee, Y. Ni and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 14319–14326 CrossRef CAS PubMed; (g) T. T. Nguyen, N.-P. T. Le, T. T. Nguyen and P. H. Tran, RSC Adv., 2019, 9, 38148–38153 RSC.
- (a) K. Ohta, Y. Inagaki-Oka, H. Hasebe and I. Yamamoto, Polyhedron, 2000, 19, 267–274 CrossRef CAS; (b) J. L. Dempsey, B. S. Brunschwig, J. R. Winkler and H. B. Gray, Acc. Chem. Res., 2009, 42, 1995–2004 CrossRef CAS PubMed; (c) F. Wang and C. Chen, Polym. Chem., 2019, 10, 2354–2369 RSC.
- For recent examples, see: (a) P. Muthupandi and G. Sekar, Tetrahedron Lett., 2011, 52, 692–695 CrossRef CAS; (b) Y. Yu, C. Lin, B. Li, P. Zhao and S. Zhang, Green Chem., 2016, 18, 3647–3655 RSC; (c) A. Saha, S. Payra and S. Banerjee, New J. Chem., 2017, 41, 13377–13381 RSC; (d) A. R. Patel, G. Patel and S. Banerjee, ACS Omega, 2019, 4, 22445–22455 CrossRef CAS PubMed.
- For recent examples, see: (a) J.-W. Xue, M. Zeng, X. Hou, Z. Chen and G. Yin, Asian J. Org. Chem., 2018, 7, 212–219 CrossRef CAS; (b) S. W. Kim, T.-W. Um and S. Shin, J. Org. Chem., 2018, 83, 4703–4711 CrossRef CAS PubMed; (c) J. Zhou, X.-Z. Tao, J.-J. Dai, C.-G. Li, J. Xu, H.-M. Xu and H.-J. Xu, Chem. Commun., 2019, 55, 9208–9211 RSC; (d) W. Yang, Y. Chen, Y. Yao, X. Yang, Q. Lin and D. Yang, J. Org. Chem., 2019, 84, 11080–11090 CrossRef CAS PubMed; (e) A. Y. Dubovtsev, N. V. Shcherbakov, D. V. Dar'in and V. Y. Kukushkin, J. Org. Chem., 2020, 85, 745–757 CrossRef CAS PubMed.
- For recent examples, see: (a) X. Liu and W. Chen, Organometallics, 2012, 31, 6614–6622 CrossRef CAS; (b) X. Zeng, C. Miao, S. Wang, C. Xia and W. Sun, RSC Adv., 2013, 3, 9666–9669 RSC; (c) J.-W. Yu, S. Mao and Y.-Q. Wang, Tetrahedron Lett., 2015, 56, 1575–1580 CrossRef CAS; (d) R. Chebolu, A. Bahuguna, R. Sharma, V. K. Mishra and P. C. Ravikumar, Chem. Commun., 2015, 51, 15438–15441 RSC; (e) J. M. Khurana, A. Lumb and A. Chaudhary, Monatsh. Chem., 2017, 148, 381–386 CrossRef CAS.
- For oxidative coupling reactions, see: (a) Q. Zhang, C.-M. Xu, J.-X. Chen, X.-L. Xu, J.-C. Ding and H.-Y. Wu, Appl. Organomet. Chem., 2009, 23, 524–526 CrossRef CAS; (b) X. Guo, W. Li and Z. Li, Eur. J. Org. Chem., 2010, 5787–5790 CrossRef CAS; (c) M. R. Rohman, I. Kharkongor, M. Rajbangshi, H. Mecadon, B. M. Laloo, P. R. Sahu, I. Kharbangar and B. Myrboh, Eur. J. Org. Chem., 2012, 320–328 CrossRef CAS; (d) Y. Su, X. Sun, G. Wu and N. Jiao, Angew. Chem., Int. Ed., 2013, 52, 9808–9812 CrossRef CAS; (e) W.-X. Lv, Y.-F. Zeng, S.-S. Zhang, Q. Li and H. Wang, Org. Lett., 2015, 17, 2972–2975 CrossRef CAS PubMed; (f) J. B. Bharate, S. Abbat, R. Sharma, P. V. Bharatam, R. A. Vishwakarma and S. B. Bharate, Org. Biomol. Chem., 2015, 13, 5235–5242 RSC; (g) P. Hirapara, D. Riemer, N. Hazra, J. Gajera, M. Finger and S. Das, Green Chem., 2017, 19, 5356–5360 RSC; (h) Y. Kumar, Y. Jaiswal and A. Kumar, Eur. J. Org. Chem., 2018, 494–505 CrossRef CAS.
- For one-pot syntheses via Sonogashira/Heck coupling–oxidation, see: (a) H. Min, T. Palani, K. Park, J. Hwang and S. Lee, J. Org. Chem., 2014, 79, 6279–6285 CrossRef CAS PubMed; (b) D. Saberi, H. Hashemi and K. Niknam, Asian J. Org. Chem., 2017, 6, 169–173 CrossRef CAS; (c) V. G. Jadhav, S. A. Sarode and J. M. Nagarkar, Tetrahedron Lett., 2017, 58, 1834–1838 CrossRef CAS; (d) P. Niesobski, I. S. Martínez, S. Kustosz and T. J. J. Müller, Eur. J. Org. Chem., 2019, 5214–5218 CrossRef CAS.
- (a) M. Palucki and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 11108–11109 CrossRef CAS; (b) B. C. Hamann and J. F. Hartwig, J. Am. Chem. Soc., 1997, 119, 12382–12383 CrossRef CAS.
- (a) W. A. Moradi and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7996–8002 CrossRef CAS PubMed; (b) S. Lee, N. A. Beare and J. F. Hartwig, J. Am. Chem. Soc., 2001, 123, 8410–8411 CrossRef CAS PubMed; (c) E. M. Vogl and S. L. Buchwald, J. Org. Chem., 2002, 67, 106–111 CrossRef CAS PubMed; (d) D. A. Culkin and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 9330–9331 CrossRef CAS PubMed; (e) R. Martín and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 7236–7239 CrossRef PubMed; (f) G. D. Vo and J. F. Hartwig, Angew. Chem., Int. Ed., 2008, 47, 2127–2130 CrossRef CAS PubMed . For a review on α-arylation, see: ; (g) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676–707 CrossRef CAS PubMed.
- (a) M. Palucki, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 3395–3396 CrossRef CAS; (b) K. E. Torraca, X. Huang, C. A. Parrish and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 10770–10771 CrossRef CAS PubMed; (c) H. Zhang, P. Ruiz-Castillo and S. L. Buchwald, Org. Lett., 2018, 20, 1580–1583 CrossRef CAS PubMed.
- 2-Dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl.
- The use of organic bases, such as Et3N and DBU resulted in virtually no reaction.
- The reaction of 1a with 2-bromopyridine led to decomposition of the starting materials.
- The attempted coupling of ethyl hydroxyacetate with 2a failed, producing a complex mixture of products. Hydroxyacetone was not suitable for the reaction.
- (a) A. S. Guram, X. Bei and H. W. Turner, Org. Lett., 2003, 5, 2485–2487 CrossRef CAS PubMed; (b) C. Berini, D. F. Brayton, C. Mocka and O. Navarro, Org. Lett., 2009, 11, 4244–4247 CrossRef CAS PubMed; (c) Q. Gao and S. Xu, Org. Biomol. Chem., 2018, 16, 208–212 RSC; (d) M. Kuriyama, S. Nakashima, T. Miyagi, K. Sato, K. Yamamoto and O. Onomura, Org. Chem. Front., 2018, 5, 2364–2369 RSC.
- Syntheses of benzophenones by the palladium-catalysed coupling of benzaldehydes with aryl halides have been reported. (a) B. Suchand and G. Satyanarayana, J. Org. Chem., 2016, 81, 6409–6423 CrossRef CAS PubMed; (b) T. Wakai, T. Togo, D. Yoshidome, Y. Kuninobu and M. Kanai, ACS Catal., 2018, 8, 3123–3128 CrossRef.
- The reaction with 1.2 equiv. 2a under air afforded 3a in 45% yield, indicating that aerobic oxidation was impractical under our conditions. See ref. 6a.
- For an account on the synthesis 1,2-diketones from α-hydroxy ketones, see: H. Liang, H. Liu and X. Jiang, Synlett, 2016, 2774–2782 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data for new compounds. See DOI: 10.1039/d0ob00575d |
| This journal is © The Royal Society of Chemistry 2020 |