Synthesis of aryl ethers of carbohydrates via reaction with arynes: selective O-arylation of trans-vicinal dihydroxyl groups in carbohydrates†
Abstract
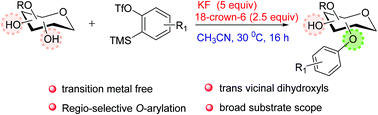
A new method for the O-arylation of carbohydrates under metal-free conditions using arynes as an aryl source has been developed. This approach works well with mono, di and trihydroxy compounds. Preferential O-arylation takes place at primary over secondary and equatorial over axial. Site-selective O-arylation was achieved with the substrate having trans vicinal diequatorial hydroxyls.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...