Copper-catalyzed direct amination of benzylic hydrocarbons and inactive aliphatic alkanes with arylamines†
Abstract
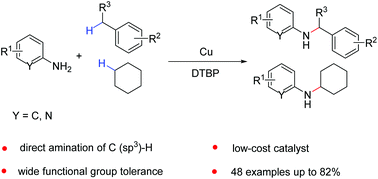
A new synthetic method toward direct C–N bond formation through saturated C–H amination of benzylic hydrocarbons and inactive aliphatic alkanes with primary aromatic amines under an inexpensive catalyst/oxidant (Cu/DTBP) system has been developed. Both aminopyridines and anilines could react smoothly with primary and secondary benzylic C–H substrates or cyclohexane to form the corresponding aromatic secondary amines in moderate to good yields. This protocol has the advantages of wide functional group tolerance and use of readily available raw materials.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...