Metal-free synthesis of imino-disaccharides and calix-iminosugars by photoinduced radical thiol–ene coupling (TEC)†
Abstract
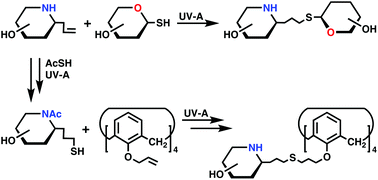
Radical thiol–ene coupling was exploited for the first time to prepare imino-disaccharides and multivalent iminosugars starting from sugar thiols and iminosugar alkenes or iminosugar thiols and tetra-allylated calixarene, respectively.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...