Newly synthesized 3-sulfenylindole derivatives from 4-hydroxydithiocoumarin using an oxidative cross dehydrogenative coupling reaction (OCDCR): potential lead molecules for antiproliferative activity†‡
Abstract
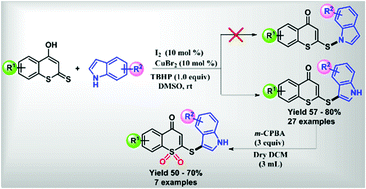
An expedient and efficient synthetic method was developed for the oxidative cross dehydrogenative coupling reaction between 4-hydroxydithiocoumarin and indole at the C-3 position regio-selectively using a combination of 10 mol% molecular iodine and TBHP in the presence of 10 mol% CuBr2 as an additive at room temperature. Mild reaction conditions, good yields and a broad substrate scope are some of the salient features of the present protocol. Additionally, the synthesized 3-sulfenylindoles derived from 4-hydroxydithiocoumarin were converted into biologically active sulfone derivatives. Interestingly, some of the compounds exhibit anti-cell proliferative activity on breast cancer (MCF-7) cells due to reactive oxygen species (ROS) mediated cell damage.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...