The effect of spermidine on guanine decomposition via photoinduced electron transfer in DNA†
Mayu
Esumi
,
Shunsuke
Sakurai
and
Makiko
Tanaka
 *
*
Department of Engineering Science, Graduate School of Informatics and Engineering, The University of Electro-Communications, 1-5-1 Chofugaoka, Chofu, Tokyo 182-8585E, Japan. E-mail: makiko.tanaka@uec.ac.jp
First published on 27th November 2019
Abstract
Spermidine, a trivalent organic cation, induced DNA structural changes and suppressed guanine photooxidative decomposition via electron transfer through pyrene-modified DNA. On the other hand, adding higher concentrations of spermidine resulted in DNA condensation. The efficiency of guanine decomposition in condensed pyrene-modified DNA was promoted remarkably.
Polyamines are present at high concentrations in all eukaryotic cells and play an important role in many biological functions. For example, spermidine, one of the naturally occurring polyamines, extends the lifespan of various types of cells.1 However, it has also been reported that the concentration of polyamines is significantly increased in cancer cells.2,3 Strategies for the inhibition of polyamine biosynthesis and transport pathways have been investigated for cancer chemotherapy.4,5 To date, the interaction between polyamines and DNA has been investigated. Double-stranded (ds) DNA can be stabilized by crosslinking with complementary strands via polyamines, in addition to the electronic interaction between cationic polyamines and the DNA phosphate site.6
It has also been reported that polyamines induce the transition of B-form DNA to A-DNA or Z-DNA by binding to the major or minor groove of DNA.7,8 On the other hand, because a high concentration of polyamines causes DNA condensation,9 the effect of polyamines on condensation has been attracting attention from the perspective of medical DNA nanotechnology such as cellular delivery.10 The condensation of long T4DNA by spermidine was observed using fluorescence microscopy.11 It was also revealed that polyamines enhanced gene expression at low concentrations but inhibited it at high concentrations.12 Such opposing effects resulted from the conformational change of DNA depending on the concentration of polyamines. In a crowded intracellular environment, noncanonical DNA structures can be stabilized, and the structural change of DNA depending on the surrounding environment is considered to regulate biological functions such as gene expression.13
Oxidative DNA damage in living organisms is regarded as a major cause of mutagenesis and carcinogenesis. Guanine is the most easily oxidizable base in DNA, and the continuous sequence of guanine is a primary target of oxidation.14 An electron hole injected into DNA by oxidation migrates through π-stacking dsDNA bases. The DNA-mediated electron transfer (ET) then generates radical cations at the guanine sites (G˙+), and oxidized products are formed by a reaction with water or oxygen.15 For decades, researchers have explored the characteristics of guanine oxidation involving DNA-mediated ET by developing synthetic DNA conjugates containing various photooxidants.16 The role of DNA-mediated ET has also been studied from the viewpoint of redox signaling between DNA-bound proteins.17,18
It is expected that DNA-mediated ET and the guanine oxidation induced by it would be sensitively affected by high concentrations of biomolecules coexisting in vivo. Recently, our group presented the first report that the efficiency of DNA-mediated ET in crowded environments formed by high concentrations of small cosolutes was different from that in dilute solution.19 In addition, it has been shown that Ψ (psi; polymer- and salt-induced)-type, liquid crystalline DNA was formed by a high concentration of poly(ethylene glycol), and guanine oxidation induced by photoinduced ET was significantly promoted in the condensed Ψ -DNA.20
Previous research has reported the inhibition of long-range ET in a linearized pUC19 plasmid condensed by spermidine.21 However, there have been no further reports on DNA-mediated ET or on DNA oxidative damage in the condensed phase. Thus, we investigated the effect of spermidine on the efficiency of guanine oxidative damage by photoinduced ET in DNA. The addition of spermidine changed the efficiency of guanine decomposition, and the decomposition significantly increased DNA condensation at high spermidine concentrations.
As in previous reports, 5-(pyrenylethynyl)-2′-deoxyuridine (PyU) in oligonucleotides was used as a hole injector in this study.19,20,22 To estimate the reduction potential (Ered) of PyU, we used pyrene-dU-CE phosphoramidite for cyclic voltammetry. Ered in DMF was obtained as −1.78 V vs. SCE in DMF (see ESI; Fig. S1†), which is considered sufficient to accept an electron from the nucleobase (for example, Eox = 1.03 V (guanine) and 1.39 V (adenine) vs. SCE)23 by photoexcitation. The LUMO (Lowest Unoccupied Molecular Orbital) level calculated at B3LYP/6-31G* for methyl-substituted PyU was −1.99 eV.24 Previously, we reported the Ered of PhU (5-(phenylethynyl)-2′-deoxyuridine) obtained by cyclic voltammetry as −2.03 V (vs. SCE) and the LUMO level as −1.55 eV.25 Thus, we confirmed that the modification of the pyrene moiety to uridine through an ethynyl linkage increases the electron-accepting ability. The relatively long absorption band of the pyrene moiety has the advantage of enabling selective excitation without irradiating DNA. The pyrene conjugated to the 5-position of uracil is located in a major groove. The DNA-mediated ET was initiated by hole injection from the excited PyU to the flanking base and induced the decomposition of distal guanines. Fig. 1 shows the DNA sequences used in this study, and melting temperatures (Tm) in the absence and presence of spermidine (0.25–50 mM) are summarized in Table 1. In DNA I, five adenines were embedded between PyU and GG in the same strand. The continuous guanine site has the lowest oxidation potential and traps a hole via DNA-mediated ET, resulting in guanine decomposition. Inosine I, an inactive guanine analog, was substituted for other guanines in DNA.26 In the sequence of DNA II, PyU and GG were adjacent to evaluate the guanine decomposition efficiency without the DNA-mediated ET process. After annealing the DNA, various concentrations of spermidine solutions were added to the aliquots.
| Spermidine | |||||||
|---|---|---|---|---|---|---|---|
| None | 0.25 mM | 0.50 mM | 2.5 mM | 5.0 mM | 30 mM | 50 mM | |
| Experimental conditions: [DNA duplex] = 4.0 μM in 50 mM Tris-HCl, pH 7.4, 100 mM NaCl. Tm is determined by monitoring the UV absorption at 260 nm. The error of Tm was less than 0.5 °C. | |||||||
| T m (DNA I), °C | 53.9 | 55.0 | 56.3 | 58.2 | 61.2 | 51.5 | 39.1 |
| T m (DNA II), °C | 54.8 | 55.3 | 55.9 | 58.4 | 60.1 | 54.1 | 34.5 |
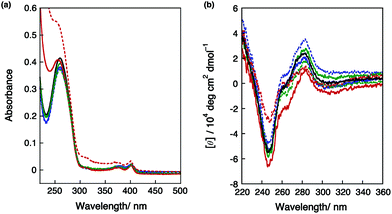
As previously reported,27 the addition of spermidine (0.25–5.0 mM) stabilized dsDNA. On the other hand, large destabilization of DNA was observed by adding high concentrations of spermidine (30 and 50 mM). Fig. 2a shows the effects of adding spermidine on the UV-vis absorption spectra of DNA I. In the buffer-salt solution containing 50 mM spermidine, an increase in optical density was observed. Fig. 2b shows the effects of adding spermidine on the CD spectra of DNA I. The first increase in the intensity of the CD band around 280 nm at 0.50 mM spermidine suggested the conversion from B-form to A-form DNA.6,28 Then, a slight decrease in the intensity of the positive band was observed at around 280 nm with 2.5–5.0 mM spermidine. At 30 mM spermidine, the decrease in the positive band could be clearly observed. By the addition of 50 mM spermidine, both the positive and negative bands of the CD spectra apparently decreased. The UV-vis and CD spectral changes showed that DNA condensation occurred at high concentrations of spermidine. It is known that DNA often forms a liquid crystalline Ψ-type condensation by adding polyamines;29,30 however, the observed CD spectral change indicates that DNA formed an aggregation state under these conditions and was not a liquid crystal structure.31
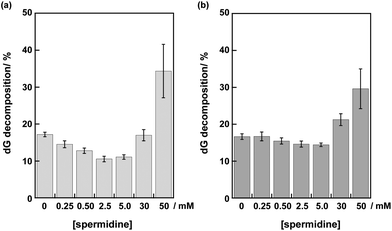
The disappearance of deoxyguanosine (dG) by irradiation with a Xe lamp through a cut-off filter (λex > 350 nm) was analyzed using HPLC after centrifugal filtration and digestion of DNA in each sample solution. The dG decomposition of DNA I by 10 min photoirradiation gradually decreased from 17 to 11% at 0–2.5 mM spermidine (Fig. 3a). The insertion of a mismatched A/C base suppressed the dG decompositions (data not shown). These results indicated that the dG decompositions in DNA I resulted from DNA-mediated ET.20 At 30 mM spermidine, the dG decomposition of DNA I apparently increased to 17%. At 50 mM spermidine, more efficient dG decomposition (34%) in DNA I was observed. The large error bar (±7%) at 50 mM spermidine was consistent with the aggregation of DNA.
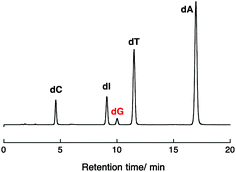
Photoirradiation experiments using DNA II containing no inserted base pairs between the PyU and the G tract were performed next to evaluate the effect of spermidine on the hole injection and trapping processes without the DNA-mediated ET process via 5AT base pairs. Because the amount of dG decomposition of DNA II by 10 min irradiation is too large to be analyzed, experiments for DNA II were performed by 1 min irradiation. As shown in Fig. 3b, the dG decompositions of DNA II by 1 min photoirradiation were slightly decreased from 17% to 14% at 0–5.0 mM spermidine. At higher concentrations of spermidine, increased guanine decomposition was observed again. It is expected that the stabilization of dsDNA by binding of spermidine affected the thermal fluctuations to form the ET-active conformation of DNA.19,32,33 At 0.25–5.0 mM concentrations, spermidine increased the rigidity of DNA. A comparison of the results for DNA I and II suggested that spermidine in the concentration range up to 5.0 mM affected the DNA-mediated ET process including forward and back electron transfer,34 resulting in the attenuation of dG decomposition. At higher concentrations of spermidine, the occurrence of large dG decomposition presumably resulted from condensation. Fig. 4 shows a representative HPLC profile of an irradiated sample solution.35 No obvious peaks of oxidized products were observed by HPLC analyses. It has been reported that in the presence of polyamines, guanine-polyamine crosslinks were produced from guanine radical cations in preference to 8-oxo-7,8-dihydroguanine;7,36 however, the yields might have been too low to be detected by HPLC.
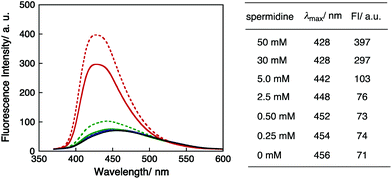
To investigate the DNA condensation state by spermidine, the fluorescence spectra of PyU in dsDNA I were recorded with varying spermidine concentrations. The excitation wavelength was 340 nm for pyrene moieties. The spectra, fluorescence maxima (λmax) and fluorescence intensities (FI) are shown in Fig. 5. The λmax values of DNA I were blue shifted as the concentration of spermidine increased, and the fluorescence intensities clearly increased at high spermidine concentrations. These results indicated that adding spermidine induced the immobilization of the pyrene moiety in DNA. The higher fluorescence intensities in the presence of high concentrations of spermidine (30 and 50 mM) are consistent with a hydrophobic environment resulting from DNA condensation.37 Note that the significant increase in fluorescence intensity and a clear increase in guanine decomposition at high concentrations of spermidine suggested that the effects of adding spermidine on the quenching of the excited pyU have been limited. There is also a possibility that the accessibility of spermidine was suppressed for DNA condensation.
The measured pH values of the 50 mM Tris-HCl sample solutions (pH 7.4) in the presence of 5, 30 and 50 mM spermidine were 8.24, 10.13, and 10.69, respectively. All three pKa values of spermidine were lower than 10.69.38 On the other hand, the measured pH values of 460 mM Tris-HCl (pH 7.2) sample solutions in the presence of 5, 30 and 50 mM spermidine were 7.34, 7.82, and 8.06, respectively. Adding spermidine in 460 mM Tris-HCl solutions showed almost no effect on the UV-vis absorption spectra of DNA I (Fig. S2†). The dG decomposition of DNA I by 10 min photoirradiation in 460 mM Tris-HCl solutions is shown in Fig. S3.† The dG decomposition was only slightly increased at 30 and 50 mM spermidine.39 Thus, the condensation and destabilization of DNA I and II obtained in 50 mM Tris-HCl buffer appear to be induced by the neutralization of spermidine resulted from deprotonation.
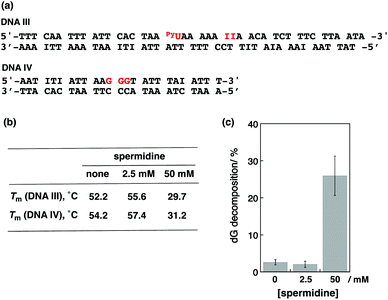
We next performed photoirradiation experiments using 2 kinds of dsDNAs. As shown in Fig. 6a, the sequence of DNA III contained the pyU site without guanine. The DNA IV sequence contained 3 guanines without PyU. Melting temperatures in the absence and presence of spermidine are summarized in Fig. 6b. The addition of 2.5 mM spermidine stabilized both dsDNAs, and the addition of 50 mM spermidine clearly destabilized both. Photoirradiation for the aliquot containing both DNA III (1 eq.) and IV (4 eq.) resulted in 2.6% dG decomposition of DNA IV in the absence of spermidine and 2.1% decomposition in the presence of 2.5 mM spermidine. On the other hand, irradiation at 50 mM spermidine induced 32% dG decomposition of DNA IV (Fig. 6c). It is considered that PyU in DNA III worked as a charge injector for DNA IV. These results suggest that not only intraduplex DNA-mediated ET but also interduplex ET between DNAs occurred in condensed form by spermidine, as well as in liquid crystalline DNA induced by poly(ethylene glycol).20
In conclusion, spermidine affected DNA structures, and the efficiency of the guanine photooxidative damage induced by DNA ET depends on the spermidine concentration. High concentrations of spermidine significantly promoted guanine oxidative decomposition in condensed DNA. These results indicate that local environmental conditions, including polyamine concentrations, would greatly affect the in vivo function of DNA ET, and oxidative damage.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank Prof. Hiroaki Kotani, Tsukuba University, for cyclic voltammetry measurements. We also wish to thank Prof. Mitsuo Shoji, Tsukuba University, for DFT calculations. This work was partly supported by the Program to Disseminate Tenure Tracking System from the MEXT, the Research Support from UEC for Young Faculty Members, and the Tobe Maki Scholarship Foundation (for M. T.).Notes and references
- T. Eisenberg, H. Knauer, A. Schauer, S. Büttner, C. Ruckenstuhl, D. Carmona-Gutierrez, J. Ring, S. Schroeder, C. Magnes, L. Antonacci, H. Fussi, L. Deszcz, R. Hartl, E. Schraml, A. Criollo, E. Megalou, D. Weiskopf, P. Laun, G. Heeren, M. Breitenbach, B. Grubeck-Loebenstein, E. Herker, B. Fahrenkrog, K. Fröhlich, F. Sinner, N. Tavernarakis, N. Minois, G. Kroemer and F. Madeo, Nat. Cell Biol., 2009, 11, 1305–1314 CrossRef CAS.
- T. J. Thomas and T. Thomas, Med. Sci., 2018, 6, 24/1–24/13 CAS.
- U. Bachrach, Amino Acids, 2004, 26, 307–309 CrossRef CAS.
- K. K. H. Vong, K. Tsubokura, Y. Nakao, T. Tanei, S. Noguchi, S. Kitazume, N. Taniguchi and K. Tanaka, Chem. Commun., 2017, 53, 8403–8406 RSC.
- N. E. Davidson, H. A. Hahm, D. E. McCloskey, P. M. Woster and R. A. Casero, Endocr. Relat. Cancer, 1999, 6, 69–73 CAS.
- M.-H. Hou, S.-B. Lin, J.-M. P. Yuann, W.-C. Lin, A. H.-J. Wang and L.-S. Kan, Nucleic Acids Res., 2001, 29, 5121–5128 CrossRef CAS.
- T. Thomas, G. D. Kulkarni, M. A. Gallo, N. Greenfield, J. S. Lewis, A. Shirahata and T. J. Thomas, Nucleic Acids Res., 1997, 25, 2396–2402 CrossRef CAS.
- E. Bignon, C. Chan, C. Morell, A. Monari, J.-L. Ravanat and E. Dumont, Chem. – Eur. J., 2017, 23, 12845–12852 CrossRef CAS.
- G. Iacomino, G. Picariello and L. D. Agostino, Biochim. Biophys. Acta, 2012, 1823, 1745–1755 CrossRef CAS.
- A. Chopra, S. Krishnan and F. C. Simmel, Nano Lett., 2016, 16, 6683–6690 CrossRef CAS.
- M. Takahashi, K. Yoshikawa, V. V. Vasilevskaya and A. R. Khokhlov, J. Phys. Chem. B, 1997, 101, 9396–9401 CrossRef CAS.
- A. Kanemura, Y. Yoshikawa, W. Fukuda, K. Tsumoto, T. Kenmotsu and K. Yoshikawa, PLoS One, 2018, 13, e0193595 CrossRef.
- H. Tateishi-karimata, K. Kawauchi and N. Sugimoto, J. Am. Chem. Soc., 2017, 140, 642–651 CrossRef.
- H. Sugiyama and I. Saito, J. Am. Chem. Soc., 1996, 118, 7063–7068 CrossRef CAS.
- X. Ming, B. Matter, M. Song, E. Veliath, R. Shanley, R. Jones and N. Tretyakova, J. Am. Chem. Soc., 2014, 136, 4223–4235 CrossRef CAS.
- H.-A. Wagenknecht, Nat. Prod. Rep., 2006, 23, 973–1006 RSC.
- A. K. Boal, J. C. Genereux, P. A. Sontz, J. A. Gralnick, D. K. Newman and J. K. Barton, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15237–15242 CrossRef CAS.
- E. O'Brien, M. E. Holt, M. K. Thompson, L. E. Salay, A. C. Ehlinger, W. J. Chazin and J. K. Barton, Science, 2017, 355, eaag1789 CrossRef.
- M. Tanaka, T. Matsumoto and H. Iida, Org. Biomol. Chem., 2018, 16, 6695–6702 RSC.
- S. Sakurai, M. Esumi and M. Tanaka, Chem. Commun., 2019, 55, 7695–7698 RSC.
- P. Das and G. B. Schuster, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 14227–14231 CrossRef CAS.
- T. Okuda, Y. Kawashima, Y. Kasahara, T. Takagi, J. Yamamoto, S. Iwai and S. Obika, Chem. Commun., 2019, 55, 14062–14065 RSC.
- K. Kawai, H. Kodera, Y. Osakada and T. Majima, Nat. Chem., 2009, 1, 156–159 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- M. Tanaka, K. Oguma, Y. Saito and I. Saito, Chem. Commun., 2012, 48, 9394–9396 RSC.
- M. A. O'Neill and J. K. Barton, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 16543–16550 CrossRef.
- Y. Terui, M. Ohnuma, K. Hiraga, E. Kawashima and T. Oshima, Biochem. J., 2005, 388, 427–433 CrossRef CAS.
- E. E. Minyat, V. I. Ivanov, A. M. Kritzyn, L. E. Minchenkova and A. K. Schyolkina, J. Mol. Biol., 1978, 128, 397–409 CrossRef.
- M. Saminathan, T. Thomas, A. Shirahata, C. K. S. Pillai and T. J. Thomas, Nucleic Acids Res., 2002, 30, 3722–3731 CrossRef CAS.
- E. Raspaud, D. Durand and F. Livolant, Biophys. J., 2005, 88, 392–403 CrossRef CAS.
- C. Li, P. Xu, Y. Gao, J. Zhang, R. Qiao and Y. Zhao, J. Phys. Chem. B, 2013, 117, 7857–7867 CrossRef CAS.
- R. N. Barnett, C. L. Cleveland, A. Joy, U. Landman and G. B. Schuster, Science, 2001, 294, 567–571 CrossRef CAS.
- M. A. O'Neill, H. C. Becker, C. Wan, J. K. Barton and A. H. Zewail, Angew. Chem., Int. Ed., 2003, 42, 5896–5900 CrossRef.
- C. Dohno, E. D. A. Stemp and J. K. Barton, J. Am. Chem. Soc., 2003, 125, 9586–9587 CrossRef CAS.
- The HPLC peak of PyU was observed around 34 min under the conditions of gradient 3–40-% acetonitrile over 40 min.
- S. Silerme, L. Bobyk, M. Taverna-Porro, C. Cuier, C. Saint-Pierre and J.-L. Ravanat, Chem. Res. Toxicol., 2014, 27, 1011–1018 Search PubMed.
- It is considered that the efficiency of hole injection by photoexcited PyU to DNA was too low to be observed by static fluorescence quenching.
- The three spermidine pKa values are reported as 8.30 ± 0.06 (pK1), 9.90 ± 0.21 (pK2) and 10.66 ± 0.21 (pK3); see: M. M. Kimberly and J. H. Goldstein, Anal. Chem., 1981, 53, 789–793 CrossRef CAS.
- The concentration of Tris-HCl buffer is already far outside the appropriate working concentration range (10–62.5 mM). Very high concentrations of Tris may have affected the experimental results.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ob01860c |
| This journal is © The Royal Society of Chemistry 2020 |