Cubic nano-silver-decorated manganese dioxide micromotors: enhanced propulsion and antibacterial performance†
Abstract
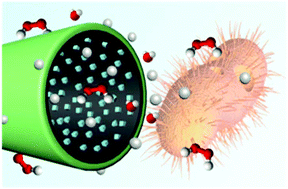
The increasing threat of antibiotic-resistant bacterial strains represents the current antibacterial dilemma and requires novel bactericidal treatment to circumvent this problem. In this work, an efficient strategy for killing bacteria using PEDOT/MnO2@Ag micromotors is reported based on the intense motion-induced convection and excellent sterilization ability of silver (Ag) ions. A distinctive inner surface structure with cubic Ag nanoparticle growth and dispersion in the MnO2 layer was constructed by simple cathodic co-electrodeposition. Due to the synergistic catalytic reaction of both MnO2 and Ag, the micromotors can rapidly swim in very low concentrations of hydrogen peroxide (H2O2). The antibacterial efficiency of the micromotors was evaluated with the Escherichia coli (E. coli) model. The continuous movement of micromotors, corresponding to violent mass transfer, along with the on-the-fly release of silver ions, greatly enhanced bacteria killing efficacy, with about 14% increase in bacterial death in 0.2% H2O2 solution as compared to no motors. Such proposed micromotors could be ideal candidates for combating antibiotic-resistant bacteria in the fields of biomedical and environmental applications.
- This article is part of the themed collections: Editor’s Choice: Recent breakthroughs in nanobiotechnology and 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...