Insights into the interparticle mixing of CsPbBr3 and CsPbI3 nanocubes: halide ion migration and kinetics†
Abstract
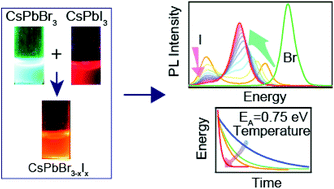
Anion exchange of CsPbX3 nanocrystals (NCs) is an easy pathway to tune the bandgap over the entire visible region. Even the mixing of pre-synthesized CsPbBr3 and CsPbI3 NCs at room temperature leads to the formation of mixed halide CsPbBr3−xIx NCs. Understanding the reaction mechanism and the kinetics of interparticle mixing is essential for fundamental aspects and device applications. Here, we probed the kinetics of ion migration through time-dependent steady-state photoluminescence (PL) spectroscopy. We found three primary PL peaks after the mixing of NCs—bromide side peak, iodide side peak, and a new peak that emerges during the reaction. The reaction follows first-order kinetics and the activation energy is 0.75 ± 0.05 eV. We propose that the free oleylammonium halides which are in dynamic equilibrium with the NCs, eventually promote interparticle mixing that follows the anion migration from the surface to the core of the nanocrystal, which is the rate-limiting step. Overall, the inherent reaction rate between the halide anions and the nanocrystals governs the reaction kinetics.


 Please wait while we load your content...
Please wait while we load your content...