Open Access Article
Open Access ArticleAmphiphilic gold nanoparticles perturb phase separation in multidomain lipid membranes†
Ester
Canepa
 a,
Sebastian
Salassi
a,
Sebastian
Salassi
 b,
Anna Lucia
de Marco
b,
Chiara
Lambruschini
b,
Anna Lucia
de Marco
b,
Chiara
Lambruschini
 a,
Davide
Odino
b,
Davide
Bochicchio
b,
Fabio
Canepa
a,
Davide
Odino
b,
Davide
Bochicchio
b,
Fabio
Canepa
 a,
Claudio
Canale
b,
Silvia
Dante
c,
Rosaria
Brescia
d,
Francesco
Stellacci
a,
Claudio
Canale
b,
Silvia
Dante
c,
Rosaria
Brescia
d,
Francesco
Stellacci
 e,
Giulia
Rossi
e,
Giulia
Rossi
 *b and
Annalisa
Relini
*b and
Annalisa
Relini
 *b
*b
aDepartment of Chemistry and Industrial Chemistry, University of Genoa, Via Dodecaneso 31, 16146 Genoa, Italy. E-mail: rossig@fisica.unige.it; relini@fisica.unige.it
bDepartment of Physics, University of Genoa, Via Dodecaneso 33, 16146 Genoa, Italy
cMaterials Characterization Facility, Italian Institute of Technology, Via Morego 30, 16163 Genoa, Italy
dElectron Microscopy Facility, Italian Institute of Technology, Via Morego 30, 16163 Genoa, Italy
eInstitute of Materials, École Polytechnique Fédérale de Lausanne, Route Cantonale, 1015 Lausanne, Switzerland
First published on 17th September 2020
Abstract
Amphiphilic gold nanoparticles with diameters in the 2–4 nm range are promising as theranostic agents thanks to their spontaneous translocation through cell membranes. This study addresses the effects that these nanoparticles may have on a distinct feature of plasma membranes: lipid lateral phase separation. Atomic force microscopy, quartz crystal microbalance, and molecular dynamics are combined to study the interaction between model neuronal membranes, which spontaneously form ordered and disordered lipid domains, and amphiphilic gold nanoparticles having negatively charged surface functionalization. Nanoparticles are found to interact with the bilayer and form bilayer-embedded ordered aggregates. Nanoparticles also suppress lipid phase separation, in a concentration-dependent fashion. A general, yet simple thermodynamic model is developed to show that the change of lipid–lipid enthalpy is the dominant driving force towards the nanoparticle-induced destabilization of phase separation.
Introduction
The study of the molecular mechanisms of nanoparticle (NP)–lipid bilayer interactions is the first step towards the understanding of the biological effects of NPs on cell membranes. A better understanding of the NP–lipid interface at the fundamental level can assist the design and engineering of NPs with a controlled interaction with the membrane biological environment. Here we focus on monolayer-protected gold nanoparticles (Au NPs) that are designed to enter living cells for diagnostic or therapeutic purposes.1,2 The NP size determines to a large extent the internalization mechanism: large NPs, with a diameter >10 nm, are usually internalized via active, endocytic pathways, while smaller NPs can access both the endocytic and the passive translocation pathway.3 In particular, small Au NPs functionalized by a mixture of hydrophobic and hydrophilic ligands have been shown to stably interact with model membranes4 and even translocate through cell membranes via energy independent mechanisms.5,6 Experimental and computational approaches have joined their efforts in the last decade to elucidate the molecular mechanisms7–10 of passive interaction between small metal NPs and model lipid bilayers, showing how all the physico-chemical characteristics of the NP–lipid interface, including NP surface chemistry,11 patterning,4,5,12–14 charge,15 and lipid composition,16,17 concur with comparable influence to determine the fate of the NP at the bilayer surface.On the lipid side of the NP–lipid interface, lipid heterogeneity is a key ingredient of any model system aiming to approach the complexity of biological membranes. In biological membranes, lipids can separate laterally into dynamic nanocompartments with distinct composition and physical properties.18 These nanocompartments, or rafts, are self-assembled and specialized nanodomains, enriched in cholesterol and glycosphingolipids (such as gangliosides).18 The lateral phase separation originating the liquid ordered phase of rafts provides different lipid and protein diffusion rates and the possibility of spatial compartmentalization and segregation; rafts are directly involved in receptor and protein trafficking processes, signal transduction, as well as endocytic and exocytic membrane translocation pathways.19 Multicomponent synthetic lipid membranes that exhibit spontaneous lipid phase separation into liquid ordered (Lo) and liquid disordered (Ld) lipid phases, on the micron scale, have long been used as synthetic models of raft-forming biological membranes. Recently, the direct imaging of both micro and nano domains in giant plasma membrane vesicles has reinforced the belief that the physical driving forces underlying phase separation in synthetic membranes are the same regulating the appearance of nanodomains in biological membranes.20–22
Despite the important role played by rafts at biological level, the study of the effects of monolayer-protected metal NPs on the phase separation of model lipid bilayers is still in its infancy. For the smallest NPs (<4 nm in diameter), the fluidity of the liquid disordered phase seems to favor spontaneous NP-membrane interactions23 and NP embedding;24 another driving force for the localization of NPs in the Ld – Lo bilayer is the minimization of the hydrophobic mismatch between the NP and the lipid phase: a behavior that may be modulated by tuning the NP size, the degree of hydrophobicity and length of its ligand shell.25,26 Larger metal NPs, which are expected to adsorb at the membrane surface without being embedded, have been also reported to affect lipid packing and membrane rigidity.27,28 On the membrane surface, the curvature has been called into play as a driving force favoring the adsorption of cationic Au NPs at the boundary between liquid ordered and disordered domains.23,29 Overall, the picture emerging from these works shows a subtle dependence of the interaction between NPs and phase separated bilayers on the NP size and surface, and on the lipid composition, structure and mechanical properties, but a comprehensive view is still lacking. And most importantly, with the available knowledge no prediction on the effect that a specific NP may have on the stability of phase separation can be attempted.
Here we consider amphiphilic Au NPs, with a diameter of 2–4 nm, functionalized by a mixture of hydrophilic (anionic) and hydrophobic ligands and study their interaction with a model multidomain bilayer whose lipid composition mimics that of the neuronal plasma membrane. Indeed, in neuronal membranes, rafts have been shown to regulate fundamental membrane functions, such as signal transduction and membrane elasticity, thus impacting the exocytosis of synaptic vesicles and the release of neurotransmitters.19,30,31
We find, by atomic force microscopy (AFM) images and quartz crystal microbalance with dissipation monitoring (QCM-D) measurements, that Au NPs stably interact with the model neuronal membranes, leading to the formation of bilayer-embedded, two-dimensional, ordered NP supra-aggregates. We show that Au NPs can suppress lipid phase separation, in a concentration-dependent fashion. We interpret this result using a simple thermodynamic model and computational simulations. We show that upon addition of a small concentration of NPs in the bilayer, the change of lipid–lipid enthalpy is the dominant driving force towards the NP-induced destabilization of phase separation. Our model can be applied to any small, bare or monolayer-protected NP with a size comparable to the membrane thickness and, based on the knowledge of the NP surface functionalization and of the membrane lipid composition, it allows to predict whether the NP will have the tendency to stabilize or destabilize the lipid lateral phase separation.
Results and discussion
Experimental amphiphilic NPs and multidomain lipid membranes
Amphiphilic Au NPs having negative surface charge (NP−) were synthesized following a one-phase protocol (see the Experimental methods and Fig. S1†). The NP− core size was estimated by transmission electron microscopy (TEM) as 2.7 ± 0.8 (σ) nm. The Au core was functionalized by a thiol mixture composed of the hydrophobic 1-octanethiol (OT) and the negatively charged 11-mercapto-1-undecanesulfonate (MUS). Nuclear magnetic resonance (NMR) measurements, after decomposition, indicate a final MUS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT ratio of 80
OT ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The NP− ζ-potential was −51 ± 5 mV, a value that assures colloidal stability. The vesicles representative of the neuronal plasma membrane (M1) are composed by 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), sphingomyelin (SM), cholesterol (chol) and ganglioside GM1 in the molar ratio DOPC
20. The NP− ζ-potential was −51 ± 5 mV, a value that assures colloidal stability. The vesicles representative of the neuronal plasma membrane (M1) are composed by 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), sphingomyelin (SM), cholesterol (chol) and ganglioside GM1 in the molar ratio DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 63
GM1 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.32,33 M1 vesicles showed a ζ-potential of −64 ± 3 mV, due to the negatively charged GM1. This composition spontaneously leads to ordered – disordered lateral phase separation in the bilayer, with ordered domains enriched in SM, cholesterol and GM1.18,29,34 Control experiments were performed on other two phase-separating membrane compositions showing ordered – disordered phase immiscibility:32,35 M2 vesicles composed of DOPC
5.32,33 M1 vesicles showed a ζ-potential of −64 ± 3 mV, due to the negatively charged GM1. This composition spontaneously leads to ordered – disordered lateral phase separation in the bilayer, with ordered domains enriched in SM, cholesterol and GM1.18,29,34 Control experiments were performed on other two phase-separating membrane compositions showing ordered – disordered phase immiscibility:32,35 M2 vesicles composed of DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol 66
chol 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio) and M3 vesicles composed of DOPC
1 (molar ratio) and M3 vesicles composed of DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC
DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol 40
chol 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
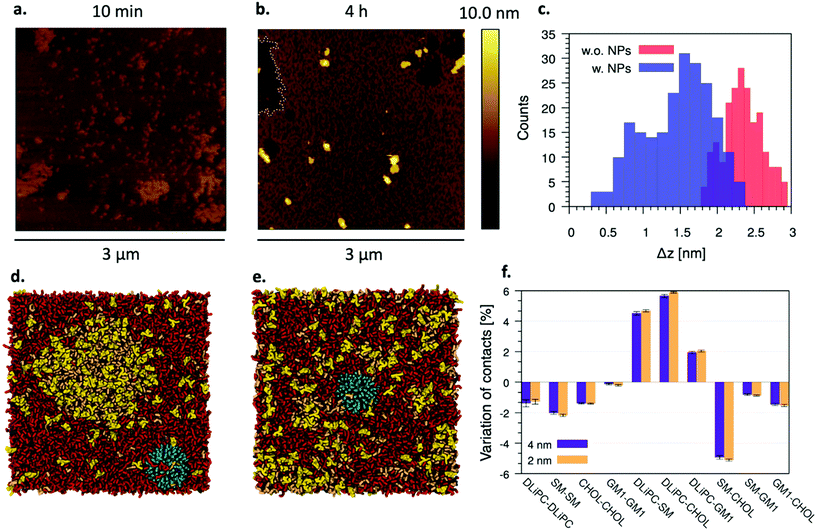
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (molar ratio), where DPPC is the saturated phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine. Multidomain vesicles were then deposited on a solid substrate, where their rupture leads to the formation of a supported lipid bilayer (SLB). Fig. 1 shows the typical morphology of our multidomain SLB for the GM1-containing neuronal membrane with composition M1. The ordered phase is well visible, with domains having regular height profiles (Fig. 1a and b). The height difference between the two phases is Δz = 2.4 ± 0.3 (σ) nm, in agreement with previous AFM measurements on GM1-containing ordered domains.32,36,37 Both the amount of large and small ordered domains and their lateral sizes display an intrinsic degree of variability within the sample, which is typical of the morphology of multidomain lipid bilayers (see also Fig. S2†). GM1-free M2 and M3 multidomain SLBs are reported in Fig. S3;† their Δz characterization is shown in Table S1 and Fig. S4.†
20 (molar ratio), where DPPC is the saturated phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine. Multidomain vesicles were then deposited on a solid substrate, where their rupture leads to the formation of a supported lipid bilayer (SLB). Fig. 1 shows the typical morphology of our multidomain SLB for the GM1-containing neuronal membrane with composition M1. The ordered phase is well visible, with domains having regular height profiles (Fig. 1a and b). The height difference between the two phases is Δz = 2.4 ± 0.3 (σ) nm, in agreement with previous AFM measurements on GM1-containing ordered domains.32,36,37 Both the amount of large and small ordered domains and their lateral sizes display an intrinsic degree of variability within the sample, which is typical of the morphology of multidomain lipid bilayers (see also Fig. S2†). GM1-free M2 and M3 multidomain SLBs are reported in Fig. S3;† their Δz characterization is shown in Table S1 and Fig. S4.†
Computational models of the amphiphilic NPs and of the multidomain lipid bilayers
Our computational model of the ligand-protected NP− has a coarse grained resolution and is compatible with the Martini coarse grained force field38 for biomolecular simulations. The model is described in detail in our previous publications.9,39 Here, we consider NP− with an Au core diameter of 2 or 4 nm, functionalized by a mixture of MUS and OT ligands, in the 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio (Fig. 2a). The in silico multidomain membranes are modeled by the Martini force field38 as well. The model neuronal plasma membrane is composed by a mixture of 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLiPC), SM, cholesterol and GM1, in the proportion DLiPC
30 ratio (Fig. 2a). The in silico multidomain membranes are modeled by the Martini force field38 as well. The model neuronal plasma membrane is composed by a mixture of 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLiPC), SM, cholesterol and GM1, in the proportion DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 56
GM1 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (Fig. 2b). The simulated M1 membrane contains a lipid with a double unsaturation and significantly more cholesterol than the M1 composition used in the experiments. This modification is necessary to obtain a stable Lo – Ld phase separation in the simulations, with Lo – Ld domains characterized by neat boundaries and stable over a long simulation time scale (about 50 μs, Fig. S5†). A snapshot of the membrane, with converged Lo – Ld phase separation, is shown in Fig. 1c. Control simulations have been performed also with the alternative composition M3, in which DOPC has been replaced by DLiPC, in the same proportion used in the experiments (DLiPC
9 (Fig. 2b). The simulated M1 membrane contains a lipid with a double unsaturation and significantly more cholesterol than the M1 composition used in the experiments. This modification is necessary to obtain a stable Lo – Ld phase separation in the simulations, with Lo – Ld domains characterized by neat boundaries and stable over a long simulation time scale (about 50 μs, Fig. S5†). A snapshot of the membrane, with converged Lo – Ld phase separation, is shown in Fig. 1c. Control simulations have been performed also with the alternative composition M3, in which DOPC has been replaced by DLiPC, in the same proportion used in the experiments (DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC
DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol 40
chol 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). The reliability of the Martini coarse grained model to look at the interaction between model anionic Au NPs and phosphatidylcholine bilayers has been thoroughly discussed in our previous works.9,11,39 We showed9 that the absence of long-range electrostatic interactions of the standard Martini model accelerates the kinetics of NP-membrane interactions, but does not alter the nature of the molecular mechanisms involved.39,40
20). The reliability of the Martini coarse grained model to look at the interaction between model anionic Au NPs and phosphatidylcholine bilayers has been thoroughly discussed in our previous works.9,11,39 We showed9 that the absence of long-range electrostatic interactions of the standard Martini model accelerates the kinetics of NP-membrane interactions, but does not alter the nature of the molecular mechanisms involved.39,40
We first aimed at verifying by QCM-D and AFM in which experimental conditions the NP− interact with the multidomain supported bilayers. We tested two alternative methods to incorporate NP− into the lipid bilayer, as described in the following sections.
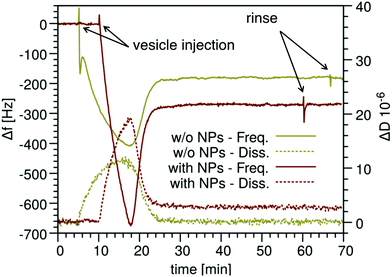
Amphiphilic NPs weakly adsorb on the SLB disordered phase
We injected the NP− in the QCM chamber after the formation of the M1 SLB. The frequency signal, shown in Fig. 3d, indicates that the NP− interact with the preformed SLB on a time scale of hours. The data confirm that the interaction is weak, leading to transient adsorption of NP− that can spontaneously desorb from the bilayer and can be completely removed by the sample upon rinsing. The maximum adsorbed mass, as calculated from the normalized frequency decrease in the first two hours, corresponds to 54 ng cm−2 of NP−. After complete NP− removal by rinsing, the bilayer is intact, as indicated by the recovery of the starting frequency. Dissipation is almost not affected by NP−, and after rinsing the bilayer is as rigid as before NP− addition, confirming that NP− are not incorporated by the bilayer but are only weakly adsorbed on top of it. In AFM experiments, we deposited different amounts of NP− on preformed multidomain M1 SLBs and waited different incubation times before imaging, as detailed in the Experimental methods and in Fig. S6.† We could not observe any NP− in images taken 40 minutes after deposition, suggesting a weak interaction of monomeric NP− with the bilayer surface. Only after several hours, it was possible to observe large NP− clusters adsorbed on the bilayers (Fig. 3a–c), presumably formed as a consequence of nanoparticle diffusion on top of the bilayer. Even according to AFM the interaction between the NP− and the M1 SLB is weak, as the NP− clusters were often dragged by the AFM tip and most NP− clusters could be removed from the bilayer by rinsing.This weak adsorption of NP− on top of the supported bilayer is coherent with the state of the art knowledge about the mechanism of interaction between MUS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT NP− and neutral phosphatidylcholine bilayers. The partial or total embedding of anionic NPs in the bilayer is characterized by large free energy barriers,8,9 which can be lowered by the presence of defects.40 The molecular mechanism allowing for the stabilization of the NP-membrane interaction requires the transient exposure of one or more hydrophobic lipid tails to the NP interface.10,41 These lipid tail protrusions are more likely to take place at bilayer edges, such as those forming at the perimeter of hole defects in SLBs.40 Our M1 SLB samples, though, are defect free on a scale of tens of μm (Fig. 3a–c and Fig. S7†). Furthermore, the physical constraints imposed by the solid support on the defect-free SLB is likely to suppress lipid dynamics in both in-plane42 and out-of-plane directions, slowing down the kinetics of NP-bilayer interactions.
OT NP− and neutral phosphatidylcholine bilayers. The partial or total embedding of anionic NPs in the bilayer is characterized by large free energy barriers,8,9 which can be lowered by the presence of defects.40 The molecular mechanism allowing for the stabilization of the NP-membrane interaction requires the transient exposure of one or more hydrophobic lipid tails to the NP interface.10,41 These lipid tail protrusions are more likely to take place at bilayer edges, such as those forming at the perimeter of hole defects in SLBs.40 Our M1 SLB samples, though, are defect free on a scale of tens of μm (Fig. 3a–c and Fig. S7†). Furthermore, the physical constraints imposed by the solid support on the defect-free SLB is likely to suppress lipid dynamics in both in-plane42 and out-of-plane directions, slowing down the kinetics of NP-bilayer interactions.
As we observed in our AFM images that the vast majority of NP− clusters are located in the disordered phase of M1 SLBs (Fig. 3a–c), we used molecular dynamics (MD) simulations to understand what is the molecular mechanisms of adsorption of the NP− on the Ld phase and to quantify the thermodynamic advantage of the Ld–NP interaction over the Lo–NP interaction. In our MD simulations, when the NP− (of 2 and 4 nm in diameter) were initially placed in the water phase, not in contact with the membrane surface, either they spontaneously adsorbed onto the Ld phase (Fig. 3e), or they transiently adsorbed onto the Lo phase and then quickly (after ∼100 ns) diffused towards the Ld phase, where they remained for the rest of the simulated time (20 μs). On the Ld phase, the NP− adsorbed more stably and closer to the membrane midplane than on the Lo phase, as disorder allows for the necessary conformational adjustments of the lipid headgroups in contact with the NP−.
We calculated the free energy of adsorption of a single NP− on the surface of the Ld and Lo phase of M1 bilayers. The potential of mean force profiles shown in Fig. 3f indicate that the Ld phase, with a binding free energy of ∼18 kJ mol−1 (∼9kBT), is favored over the Lo phase (∼11 kJ mol−1, ∼5kBT). This substantial difference between the two phases agrees with the experimental results.
Membrane fluctuations influence NP uptake
The slow and relatively weak adsorption of NP− onto the preformed M1 bilayer is consistent with the previous experimental observation of little to no absorption of anionic Au NPs injected on GM1-containing multidomain SLBs of slightly different composition.29 On the other hand, the same anionic MUS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT Au NPs are known to interact stably with neutral phosphatidylcholine vesicles4,43 and with multicomponent vesicles containing significant percentages of DOPC and SM.24 Our previous computational results also indicate that the spontaneous incorporation of anionic MUS
OT Au NPs are known to interact stably with neutral phosphatidylcholine vesicles4,43 and with multicomponent vesicles containing significant percentages of DOPC and SM.24 Our previous computational results also indicate that the spontaneous incorporation of anionic MUS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT Au NPs in free standing, liquid disordered phosphatidylcholine bilayers9,11,39 is possible, though characterized by slow kinetics. Membrane fluctuations and unrestrained lipid diffusion in the vesicle bilayer allow for the transient defects that stabilize the contact with the anionic NP and, if the NP size permits it, also allow for the incorporation of the anionic NP into the bilayer core.
OT Au NPs in free standing, liquid disordered phosphatidylcholine bilayers9,11,39 is possible, though characterized by slow kinetics. Membrane fluctuations and unrestrained lipid diffusion in the vesicle bilayer allow for the transient defects that stabilize the contact with the anionic NP and, if the NP size permits it, also allow for the incorporation of the anionic NP into the bilayer core.
To overcome the motional constraints imposed on the membrane by the solid support and yet exploit AFM imaging and QCM-D investigation for the characterization of a stable NP-bilayer complex, we devised a second experimental set-up in which NP− are pre-incubated with multidomain lipid vesicles for a variable lag of time. After pre-incubation, the vesicles are deposited on the substrate (mica or the QCM-D sensor), where they rupture and form a SLB with embedded NP− (see the Experimental methods and Fig. S6†). Utilizing the QCM-D technique, we verified the uptake of NP− by the bilayer of pre-incubated M1 vesicles, as shown in Fig. 4. The QCM traces indicate the adhesion of a larger mass in the case of pre-incubated vesicles than without NP− during the entire process of SLB formation. The frequency shift when SLBs are formed corresponds to Δm = 267 ± 18 ng cm−2. Interpreting this difference as the mass of the embedded NP−, we calculated that the number of embedded NP− is ≈0.017 NP− nm−2. Since for the lipids we obtained a value of 1.34 molecule nm−2, the calculated ratio between the number of lipid molecules and the number of NP− is ∼80 lipids/NP− (see the ESI† for details). Such a large NP− uptake is confirmed by AFM imaging, which we will discuss in detail in the next sections. To further confirm the role of membrane fluctuations in favoring the full embedding of NP− into the M1 bilayer, we monitored by QCM-D and AFM imaging the NP− uptake in a control membrane with a larger mechanical rigidity. M2 bilayers, which differ from M1 membranes only in the removal of the GM1 component, are expected to have a stiffer disordered phase than M1 bilayers.37,44 After the same pre-incubation time of M2 vesicles with NP−, both QCM-D and AFM data, reported in Fig. S8,† confirm that the M2 composition shows a lower NP− uptake than the M1 composition.
Now that we have devised an experimental protocol assuring a spontaneous, consistent and stable uptake of NP− into the M1 membranes, we can proceed with the investigation of the effects of NP− absorption on the properties of the multidomain bilayer.
Amphiphilic NPs alter the membrane phase separation
Fig. 5a and b show the AFM images of M1 SLBs formed after vesicle pre-incubation with NP− for 10 min and 4 h, respectively. Contrary to what happens when preformed SLBs are incubated with NP− (Fig. 3a–c), only 10 minutes of vesicle pre-incubation with NP− are sufficient to alter the shape and compactness of ordered domains, which here have irregular shapes and ragged boundaries, with an overall morphology dramatically different from that observed in the absence of NP− (Fig. 1a and b). The distribution of the height difference (Δz) between ordered and disordered domains is way broader than without NP− and shifts from Δz = 2.4 ± 0.3 (σ) nm (without NP−) to Δz = 1.5 ± 0.5 (σ) nm after 10 min of pre-incubation, as shown by the histograms in Fig. 5c. When the NP− are pre-incubated for longer times (4 h in Fig. 5b), the ordered – disordered phase separation vanishes. Here, it is possible to identify sparse disordered patches and few NP− clusters, but most of the sample is characterized by a new rough phase containing both lipids and NP−. The structural features of this mixed NP-lipid phase will be discussed in more detail in the next sections.Our MD simulations also show that after the spontaneous adsorption of the NP− on the Ld phase (Fig. 5d), the Lo – Ld phase separation is altered and no clear Lo domain can be detected anymore (Fig. 5e, ∼20 μs later). We observe the disappearance of the Lo – Ld phase separation independently of the NP− size (2 or 4 nm in core diameter), and on the degree of NP− penetration into the membrane core. The mixing of the lipid species in the M1 bilayer can be quantified by means of lipid–lipid contacts; Fig. 5f shows the variation of lipid–lipid contacts in the simulated bilayer upon the incorporation of a single NP−. At equilibrium, the contacts between the unsaturated lipid, DLiPC, and the lipids that enrich the Lo phase (SM, cholesterol, and GM1) increase at the expense of all intra-domain contacts. The adsorption of the NP− on a bilayer with the same composition, but no lateral phase separation, is thermodynamically favored with respect to the adsorption on both the Ld and Lo phases, as shown by the free energy profile of adsorption reported in Fig. S9.†
Here, both AFM imaging and MD simulations indicate that the NP− can alter the membrane phase separation of the model neuronal membrane with composition M1.
In the next section, we propose a simple thermodynamic model to explain this behavior.
NPs disrupt phase separation by altering lipid–lipid interactions
In lipid bilayers, Lo – Ld spontaneous phase separation results from the minimization of Gibbs free energy. A lipid mixture that, at equilibrium, is found in the Lo – Ld coexistence state is such that:| ΔGS→M = ΔHS→M − TΔSS→M > 0 | (1) |
The transformation from a phase separated bilayer (state S) to a mixed bilayer (state M) implies an enthalpic penalty (usually referred to as “enthalpy of mixing”) and an entropic gain.45 The entropic term comprises both lipid configurational entropy and mixing entropy, the latter being the most significant. In the following, we will omit the S → M subscript for the sake of notation simplicity, but all energy differences will refer to an S → M transformation. We want to analyze the situation in which a phase separated membrane hosts a certain concentration of NP inclusions. We assume that the partitioning of the inclusions into the membrane hydrophobic core is favorable from a thermodynamic point of view, that is to say, we assume that the NPs penetrate the membrane core spontaneously. Once inside the membrane, the sign of ΔGNP will determine whether the NPs cause lipid mixing or not:
| ΔGNP = ΔHNP − TΔSNP | (2) |
1. ΔH > ΔHNP. In this case, the presence of NPs embedded in the Ld domain of the phase separated bilayer reduces the stability of the phase separated state. We expect the term ΔHNP to depend on cNP. There may be a threshold in the NP concentration, ctr, at which the mixed state becomes equally favorable than the phase separated state (ΔHNP = TΔSNP). For concentrations higher than ctr, the mixed state is favored over the phase separated state. This seems to be the case for the NP− in the M1 bilayer, in which phase separation is no more observed when a large number of NP− are embedded in the bilayer during pre-incubation (Fig. 4 and Fig. 5a and b);
2. ΔHNP > ΔH. This means that the presence of NPs stabilizes phase separation. This stabilization may be due to different NP-lipid interactions. NPs may for example accumulate at phase boundaries driven by hydrophobic mismatch, reducing line tension at the Lo – Ld interface.23 Or, NPs may have extremely favorable dispersion interactions with lipids in the Ld phase, such as fullerenes and other small hydrophobic molecules.46,47
Relatively short equilibrium MD simulations allow for the explicit calculation of the enthalpic terms in eqn (1) and (2), as detailed in the ESI.† In this way, MD can be effectively used as a predictive tool to assess the propensity of NPs of different composition to alter or stabilize phase separation in multicomponent lipid bilayers. We thus calculated ΔH and ΔHNP for NP− in M1 bilayers. We found that indeed ΔH > ΔHNP, and conclude that the NP− inclusion in the bilayer core disfavors phase separation by altering the enthalpic gain associated with lipid–lipid interactions within Ld domains (Table 1). We also tested this approach on two control cases, in which both the NP surface chemistry and the bilayer composition were varied. We used hydrophobic nanoparticles and M3 membranes, as detailed in the ESI (Tables S2 and S4†). The model correctly predicts the effect of NPs on lipid lateral phase separation for both controls, as confirmed by experimental data taken from this work and from the literature.
| Membrane composition | ΔH S→M [kJ mol−1] | NP type (NP/lipid molar ratio in simulation) | ΔH NP S→M [kJ mol−1] | Model prediction | Exp. validation |
M1 DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 56 GM1 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 2850 lipids 9 2850 lipids |
+9.96 103 | NP−(0.35 10−3) | −1.2 103 | destabilize phase separation already at low concentration | This work |
Amphiphilic NPs form ordered supramolecular aggregates within the membrane
A more in-depth investigation of the new rough phase observed after pre-incubation of M1 bilayers with NP− for 4 h (Fig. 5b) revealed membrane patches displaying ordered NP− lattices. This behavior is completely absent after NP− incubation on preformed SLB (Fig. 3a–c and Fig. S7†). Fig. S12a† shows a representative image of these peculiar patches already after pre-incubation for 10 min. With a pre-incubation of only a few minutes, these lattices are isolated and not widespread. They become much more evident and uniformly distributed throughout the bilayer when the pre-incubation time is increased to a few hours. Fig. 6a shows the periodic NP− lattice observed after pre-incubation for 4 h. The supramolecular aggregate is quite regular, as shown by the digital zoom in Fig. 6a in which an ordered lattice is recognizable. The periodicity of the NP− lattice is highlighted also by the height profile of a NP− row and the Fourier transform shown in Fig. S12c and d,† respectively. The NP–NP distance is equal to 7.5 ± 0.1 nm, as shown by the histogram in Fig. 6b. The standard deviation, 1.6 nm, of NP–NP distances is consistent with the NP− size distribution. In addition, we measured the height difference Δz between these supramolecular aggregates and the disordered phase, by sampling the height step along the contour of disordered patches located within the widespread lattice (e.g., the one outlined by the dashed white contour in Fig. 5b). The histogram of Δz is shown in Fig. 6c and it reveals that the NP− are at least partially embedded into the bilayer, since Δz = 1.2 ± 0.3 (σ) nm, way below the mean NP− diameter (Fig. S13†).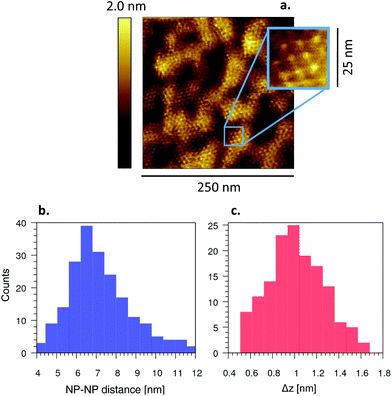 | ||
| Fig. 6 Supramolecular lattice formed by M1 bilayer-embedded NP−. a Widespread NP− lattice, imaged by AFM, after 4 h of pre-incubation with NP− (20 μL, 0.12 mg mL−1). Digital zoom of the area with blue contour showing the lattice order at higher magnification. b Distribution of NP–NP distances. c Distribution of the height of the supramolecular lattice, Δz, measured with respect to the disordered phase. Δz data have been collected along the contour of the sparse disordered patches located within the widespread NP− lattice (e.g., the one outlined by the white dashed line in Fig. 5b). | ||
In order to assess the thermodynamic stability of NP− aggregates within the M1 bilayer, and characterize NP− aggregation with molecular resolution, we performed simulations in which two or more NP− were embedded in the bilayer. In unbiased simulations NP− with a diameter of 2 nm form transient dimers within the simulation time scale (10 μs). On the contrary, two NP− with a diameter of 4 nm spontaneously form a stable dimer, as shown in Fig. 7a and b. From a thermodynamic point of view, dimerization appears to be highly favored for 4 nm NP− (Fig. 7c). The fine size dependence of NP-bilayer interactions is expected in this specific system.43 As a rule of thumb one may expect that, if NP aggregation is membrane-driven, it may be observed only for NPs whose size is comparable to or larger than the membrane thickness. Indeed, the characterization of membrane deformation when small, 2 nm NP− are embedded in the bilayer indicate little to no perturbation of the bilayer structure; on the contrary, NP− with a diameter of 4 nm can protrude significantly out of the bilayer. The NP− we consider (2 and 4 nm in diameter) are representative of the range of sizes of the experimental sample (Fig. S13†), and we speculate that the transition from the non-aggregation to the aggregation regime happens in between 2 and 4 nm in diameter. An in-depth exploration of this effect will deserve our attention in future work. Here we investigate in detail the structural features of the dimer formed by the 4 nm NP−. In between the NP− forming the dimer, the charged MUS ligands lead to the formation of a small nanopore,16 containing both counterions and water molecules (see Fig. 7b). Once formed, the pore is stable for several microseconds and does not grow in size, so that no further membrane damage has been observed. The formation of nanopores at the interface between adjacent NP− allows the ligands to re-orient all around the NP−. The different configurations adopted by the ligands when the NP− is embedded in the membrane in the monomeric and dimer state are shown in Fig. S14.†
After having proved the spontaneous tendency to dimerization, we continued the investigation of NP− aggregation using bigger systems containing several NP− embedded in the bilayer. As a first test, we started with a preformed hexagonal lattice containing 7 NP− (see Fig. S15†) and we verified by unbiased MD simulations that the aggregate is stable for 10 μs. During this MD run small pores spontaneously formed between the NP−, as already observed for dimers. We also used a starting configuration (Fig. 7d) in which 7 NP− do not interact with each other, with the aim to assess if an ordered lattice can spontaneously form. After ∼23 μs of unbiased run, the NP− form a stable hexagonal aggregate (see the Movie S1†), in which the NP–NP distance nicely match the experimental data (first neighbor peak at 7.5 nm, shown in the inset of Fig. 7c). Once again, in between the NP− forming the hexagonal aggregate we can observe the spontaneous formation of small pores. On the microsecond time scale, nanopores can open and close several times, occupying in turn the interstitial space between different pairs of NP−.
Fig. 7f shows the protrusion of a single NP− and of a NP− dimer on top of the bilayer obtained from unbiased MD simulations. In both experiments and simulations, the NP− average height over the bilayer is way below the NP− diameter. This is coherent with a picture in which the NPs are partially embedded into the bilayer. More quantitatively, the slight difference of the height difference Δz between NP− and the disordered phase in simulations and in experiments (average protrusion of 1.5 nm in the simulations vs. 1.2 nm in the experiments, see Fig. 6c) can be attributed to the size dispersion of NP− in the experimental sample.
Materials and methods
The experimental uncertainties reported in the paper refer to standard errors. When the standard deviation (σ) is used, it is explicitly indicated.Experimental methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT ligand ratio, 1H NMR analysis was performed before and after decomposition of the gold core, respectively. The colloidal stability of NP− dispersions was assessed by ζ-potential measurements.
OT ligand ratio, 1H NMR analysis was performed before and after decomposition of the gold core, respectively. The colloidal stability of NP− dispersions was assessed by ζ-potential measurements.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, 16
1), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), sphingomyelin (SM) (brain, porcine), and ganglioside GM1 (brain, ovine – sodium salt) were purchased from Avanti Polar Lipids. Cholesterol (chol), chloroform (≥99.5%), and methanol (≥99.9%) were purchased from Sigma Aldrich. All chemicals were used without further purification; all aqueous solutions were prepared using water purified with a Milli-Q ultrapure water system (MilliPore).
0), sphingomyelin (SM) (brain, porcine), and ganglioside GM1 (brain, ovine – sodium salt) were purchased from Avanti Polar Lipids. Cholesterol (chol), chloroform (≥99.5%), and methanol (≥99.9%) were purchased from Sigma Aldrich. All chemicals were used without further purification; all aqueous solutions were prepared using water purified with a Milli-Q ultrapure water system (MilliPore).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 63
GM1 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, DOPC
5, DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol 66
chol 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and DOPC
1, and DOPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPC
DPPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol 40
chol 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. These molar compositions are referred to as M1,32 M232 and M3,35 respectively. Only the M1 GM1-containing composition is representative of the neuronal plasma membrane. M2 and M3 compositions were used for control experiments. We used multidomain vesicles to prepare multidomain SLBs for both AFM and QCM-D investigations. Briefly, stoichiometric quantities of the four components dissolved in chloroform
20. These molar compositions are referred to as M1,32 M232 and M3,35 respectively. Only the M1 GM1-containing composition is representative of the neuronal plasma membrane. M2 and M3 compositions were used for control experiments. We used multidomain vesicles to prepare multidomain SLBs for both AFM and QCM-D investigations. Briefly, stoichiometric quantities of the four components dissolved in chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (2
methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) were mixed and then dried under gentle nitrogen flux. The solvent evaporation was performed at 60 °C, above the transition temperature of all the lipids in the mixture. The dried lipid film was stored one day under mild vacuum and resuspended in water at a lipid concentration of 0.5 mg mL−1. For QCM-D measurements, the film was hydrated to a lipid concentration of 1 mg mL−1 in phosphate buffered saline (PBS; 1×) to help the merge of the vesicles on the sensor of the QCM-D chamber.49 The lipid suspension was first sonicated for 15 min at 60 °C and then extruded 11 times at the same temperature to form unilamellar vesicles. Extrusion was performed using the Avanti Mini-Extruder (Avanti Polar Lipids) with a 100 nm pore size polycarbonate membrane. All vesicle suspensions were stored in a fridge at 4 °C and used within few days. Vesicle size distribution and colloidal stability were measured by DLS and ζ-potential measurements, respectively.
1, v/v) were mixed and then dried under gentle nitrogen flux. The solvent evaporation was performed at 60 °C, above the transition temperature of all the lipids in the mixture. The dried lipid film was stored one day under mild vacuum and resuspended in water at a lipid concentration of 0.5 mg mL−1. For QCM-D measurements, the film was hydrated to a lipid concentration of 1 mg mL−1 in phosphate buffered saline (PBS; 1×) to help the merge of the vesicles on the sensor of the QCM-D chamber.49 The lipid suspension was first sonicated for 15 min at 60 °C and then extruded 11 times at the same temperature to form unilamellar vesicles. Extrusion was performed using the Avanti Mini-Extruder (Avanti Polar Lipids) with a 100 nm pore size polycarbonate membrane. All vesicle suspensions were stored in a fridge at 4 °C and used within few days. Vesicle size distribution and colloidal stability were measured by DLS and ζ-potential measurements, respectively.
The data were interpreted in the assumption of rigid film formation; in this case, the Sauerbrey equation describes the relationship between Δf and mass variation (Δm): Δm = −CΔf, with C = 17.8 ng cm−2 Hz−1 (for a quartz crystal oscillating at 5 MHz).
Computational methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 56
GM1 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 modeled by the Martini coarse grained force field.50,51 For GM1 we used the topology proposed by Dasgupta et al.:52 this parametrization combines the bonded parameters by López et al.53 with the non-bonded parameters by Gu et al.54 In this description, the tendency of GM1 to self-aggregation shows a good agreement with the experimental data and it does not suffer from strong clustering behavior as observed with alternative topologies. For the other lipids the standard Martini topology is used.50,55 The membrane reference system consists of 2850 lipids solvated by approximately 35 coarse grained water beads per lipid and salt at a physiological concentration (150 mM); the final system size is 30 × 30 × 18 nm3. We started from a preformed multidomain membrane generated using the insane.py script56 (see the ESI† for details). Then, the lipids diffuse from the Lo phase to the Ld phase and vice versa until the convergence of the number of contacts between the different types of lipids is reached (about 20 μs). The system is then simulated at equilibrium for 30 μs. The NP− are inserted in the aqueous phase of the equilibrated system resulting in the different configurations presented in the Table S9† together with all relevant simulation parameters. All the MD simulations were performed with Gromacs v. 2018.57
9 modeled by the Martini coarse grained force field.50,51 For GM1 we used the topology proposed by Dasgupta et al.:52 this parametrization combines the bonded parameters by López et al.53 with the non-bonded parameters by Gu et al.54 In this description, the tendency of GM1 to self-aggregation shows a good agreement with the experimental data and it does not suffer from strong clustering behavior as observed with alternative topologies. For the other lipids the standard Martini topology is used.50,55 The membrane reference system consists of 2850 lipids solvated by approximately 35 coarse grained water beads per lipid and salt at a physiological concentration (150 mM); the final system size is 30 × 30 × 18 nm3. We started from a preformed multidomain membrane generated using the insane.py script56 (see the ESI† for details). Then, the lipids diffuse from the Lo phase to the Ld phase and vice versa until the convergence of the number of contacts between the different types of lipids is reached (about 20 μs). The system is then simulated at equilibrium for 30 μs. The NP− are inserted in the aqueous phase of the equilibrated system resulting in the different configurations presented in the Table S9† together with all relevant simulation parameters. All the MD simulations were performed with Gromacs v. 2018.57
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio, and their coarse grained Martini model is described in our previous publications.9,39
30 ratio, and their coarse grained Martini model is described in our previous publications.9,39
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OT 70
OT 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 NP− on the Lo (DLiPC
30 NP− on the Lo (DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 1
GM1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38), Ld (DLiPC
38), Ld (DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 72
GM1 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and homogeneous (DLiPC
6) and homogeneous (DLiPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SM
SM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chol
chol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GM1 56
GM1 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, with no Lo – Ld domains) phase were calculated using, as a reaction coordinate, the vertical distance between the center of mass (COM) of the membrane and the COM of the NP−. The umbrella harmonic potential had a spring constant of 1000 kJ mol−1 nm−2. For each phase, windows of 0.15 nm width were used to sample the distance in the range 5–8 nm, for a total simulated time of about 12 μs and 24 μs for the equilibration and production runs, respectively. The starting configuration consisted of a NP− placed above the membrane, in the water phase. The membrane had either the composition of the Lo, Ld or homogeneous phase, and contained 574, 574 and 1346 lipids, respectively. Each lipid phase was equilibrated for 1 μs in order to ensure the membrane equilibrium. The lipid composition in the Lo and Ld phase were obtained with an in-house script relying on python MDAnalysis libraries.61,62 The script is freely available upon request. The NP-membrane complex was solvated with coarse grained water, counter ions and physiological salt solution.
9, with no Lo – Ld domains) phase were calculated using, as a reaction coordinate, the vertical distance between the center of mass (COM) of the membrane and the COM of the NP−. The umbrella harmonic potential had a spring constant of 1000 kJ mol−1 nm−2. For each phase, windows of 0.15 nm width were used to sample the distance in the range 5–8 nm, for a total simulated time of about 12 μs and 24 μs for the equilibration and production runs, respectively. The starting configuration consisted of a NP− placed above the membrane, in the water phase. The membrane had either the composition of the Lo, Ld or homogeneous phase, and contained 574, 574 and 1346 lipids, respectively. Each lipid phase was equilibrated for 1 μs in order to ensure the membrane equilibrium. The lipid composition in the Lo and Ld phase were obtained with an in-house script relying on python MDAnalysis libraries.61,62 The script is freely available upon request. The NP-membrane complex was solvated with coarse grained water, counter ions and physiological salt solution.
For what concerns the PMF profile of the NP− dimerization in the membrane, only the homogeneous phase was considered. The reaction coordinate was the distance between the NP− COM along the membrane plane and an umbrella potential with a spring constant of 400 kJ mol−1 nm−2 was used. To generate the PMF, 11 windows were used in the range 7–9 nm and 19 windows in the range 9–14.8 nm, for a total simulated time of 1.5 μs and 40 μs for the equilibration and production runs, respectively. In the starting configurations two NP− were placed inside the membrane at a given distance between them, performing a pre-equilibration of 50 ns, before starting the umbrella sampling window simulation. In that case, a box of about 31 × 31 × 13.5 nm3 with a membrane made of 2978 lipids, solvated with coarse grained water, Na+ counter ions, and physiological salt solution (150 mM), was previously generated and pre-equilibrated. All the PMF profiles and the associated error estimation via bootstrapping of trajectories were computed with the Gromacs tool wham.63
Conclusions
We have shown, by means of AFM and QCM-D, that small, amphiphilic, and negatively charged Au NPs (NP−) can significantly perturb the lipid lateral organization of a model neuronal plasma membrane. These NP−, which have the peculiar capability to enter the cell via passive plasma membrane translocation,14 can alter the stability of ordered domains, leading to their suppression, and form stable, widespread supramolecular aggregates in which NP− are organized in ordered, two-dimensional lattices comprising NP− and lipids.The NP− studied in this work disfavor phase separation in a concentration-dependent fashion: when the uptake is very large, as in our model neuronal membranes M1, the ordered – disordered phase separation vanishes. We have provided a simple thermodynamic model to interpret the effect of NP− on lipid phase separation. The model shows that the lipid–lipid enthalpy difference between the phase-separated and the mixed bilayer, with and without NPs, is the main physical indicator of the effect that the NP will have on lipid phase separation. The model is quite general: we tested it on three different NP-bilayer compositions and it can be applied to any NP embedded in the bilayer core and with a size comparable to the thickness of the bilayer. MD simulations can be used as effective tools to predict whether a specific NP will have the tendency to disrupt or stabilize the ordered – disordered phase separation.
We envisage that the same approach could be used to investigate the effects of other membrane inclusions, such as transmembrane peptides and proteins, on the lateral heterogeneity of lipid membranes.64,65 Among transmembrane proteins one can find examples of both raft-stabilizing66,67 and raft-destabilizing proteins.68 These two opposite behaviors may also belong to the same protein in slightly different lipid environments. Gramicidin-A, for example, is a hydrophobic helical peptide that has been shown to partition in the disordered domain of DOPC/DSPC/cholesterol mixtures and then, depending on the relative lipid composition, stabilizes or destabilizes lipid lateral phase separation.68
We also investigated in detail the formation of ordered NP− aggregates within the bilayer. The interaction of the single NP− with lipids is favored by hydrophobic contacts, and this explains the spontaneous incorporation of the monomeric NP− in the bilayer.8,14 When a single NP− is embedded in the bilayer, though, its charged ligands are strongly confined to the polar interface of the membrane. Ligand flexibility is a peculiar feature of these monolayer-protected NPs. Thanks to the large flexibility of thiol ligands, the NP is able to adapt to different environments by exposing more or less hydrophobic surface to the surrounding. When embedded (or snorkeled) in the bilayer, the NP adopts a roughly cylindrical shape exposing its lateral surface, which is made of hydrophobic carbon chains, to the lipid tails, and its hydrophilic basis, covered by the sulfonate ligand terminals, to the water phase. Such a configuration closely resembles that of many transmembrane proteins. Taking the comparison further, the physical factors determining protein–protein aggregation in membranes are present also in the case of amphiphilic Au NPs. The presence of a hydrophobic mismatch between the protein (or NP) and the hosting lipid domain can cause protein (or NP) aggregation.67,69 Protein- (or NP-) induced membrane curvature can also cause aggregation as a result of the minimization of membrane deformation. In the case of NPs, ligand flexibility, which is large compared to protein conformational flexibility, concur to stabilize NP aggregates: after NP–NP aggregation has taken place, the ligands re-orient all around the NP− and ion-stabilized transient water nanopores form in the interstices between adjacent NP− surfaces. An in-depth analysis of the different driving forces causing the aggregation of NP− in phosphocholine bilayers will be the object of future work.
We speculate that the combination of the NP targeting potential and the high degree of order of these NP aggregates within the membrane may be exploited also for sensing purposes, in vitro. The self-assembly of NPs in the bilayer environment may be exploited for the creation of new hybrid materials containing ordered two-dimensional NP lattices with selective response to electromagnetic radiation. As for NP applications in biomedicine, our data provide yet another evidence that the specific physical and chemical features of the nano-bio interface, involving both the NP surface functionalization and the bilayer composition, are crucial issues in shaping the nano-bio interaction. More efforts need to be devoted to the understanding of the molecular details of such interaction, also by means of combined experimental and computational approaches, towards the rational design of NPs as drug vectors, transducers, or imaging probes.
Author contributions
EC and SS contributed equally to the work. GR and AR conceived the idea. EC performed the nanoparticle synthesis, membrane preparation, DLS characterization and most of the AFM imaging and contributed to the MUS synthesis. SS performed most of the simulations. ALdM and DB performed the remaining of the computational work. DO and CC contributed to AFM imaging. SD performed the QCM-D measurements. CL performed all NMR characterizations and supervised the MUS synthesis. RB performed the TEM imaging. GR, AR, FC, CC, FS and DB supervised the different stages of the computational and experimental work. All authors discussed the results. GR, SS, EC and AR wrote the manuscript with contributions by all other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
GR acknowledges funding from the ERC Starting Grant BioMNP 677513. EC thanks Manuela De Franco for her precious contributions to nanoparticle and MUS syntheses.Notes and references
- P. Pengo, M. Şologan, L. Pasquato, F. Guida, S. Pacor, A. Tossi, F. Stellacci, D. Marson, S. Boccardo, S. Pricl and P. Posocco, Eur. Biophys. J., 2017, 1–23 Search PubMed.
- S. Rana, A. Bajaj, R. Mout and V. M. Rotello, Adv. Drug Delivery Rev., 2012, 64, 200–216 Search PubMed.
- Y. Jiang, S. Huo, T. Mizuhara, R. Das, Y. Lee, S. Hou, D. F. Moyano, B. Duncan, X. Liang and V. M. Rotello, ACS Nano, 2015, 9, 9986–9993 Search PubMed.
- R. C. Van Lehn, P. U. Atukorale, R. P. Carney, Y.-S. Yang, F. Stellacci, D. J. Irvine and A. Alexander-Katz, Nano Lett., 2013, 13, 4060–4067 Search PubMed.
- A. Verma, O. Uzun, Y. Hu, Y. Hu, H.-S. Han, N. Watson, S. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7, 588–595 Search PubMed.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 Search PubMed.
- R. C. Van Lehn and A. Alexander-Katz, J. Phys. Chem. A, 2014, 118, 5848–5856 Search PubMed.
- R. C. Van Lehn and A. Alexander-katz, PLoS One, 2019, 14, e0209492 Search PubMed.
- S. Salassi, F. Simonelli, D. Bochicchio, R. Ferrando and G. Rossi, J. Phys. Chem. C, 2017, 121, 10927–10935 Search PubMed.
- R. C. Van Lehn and A. Alexander-Katz, Soft Matter, 2015, 11, 3165–3175 Search PubMed.
- S. Salassi, E. Canepa, R. Ferrando and G. Rossi, RSC Adv., 2019, 9, 13992–13997 Search PubMed.
- P. Gkeka, L. Sarkisov and P. Angelikopoulos, J. Phys. Chem. Lett., 2013, 4, 1907–1912 Search PubMed.
- R. C. Van Lehn and A. Alexander-Katz, Soft Matter, 2014, 10, 648–658 Search PubMed.
- S. Sabella, R. P. Carney, V. Brunetti, M. A. Malvindi, N. Al-Juffali, G. Vecchio, S. M. Janes, O. M. Bakr, R. Cingolani, F. Stellacci and P. P. Pompa, Nanoscale, 2014, 6, 7052–7061 Search PubMed.
- A. Chompoosor, G. Han and V. M. Rotello, Bioconjugate Chem., 2008, 19, 1342–1345 Search PubMed.
- P. Angelikopoulos, L. Sarkisov, Z. Cournia and P. Gkeka, Nanoscale, 2017, 9, 1040–1048 Search PubMed.
- F. Lolicato, L. Joly, H. Martinez-Seara, G. Fragneto, E. Scoppola, F. Baldelli Bombelli, I. Vattulainen, J. Akola and M. Maccarini, Small DOI:10.1002/smll.201805046.
- E. Sezgin, I. Levental, S. Mayor and C. Eggeling, Nat. Rev. Mol. Cell Biol., 2017, 18, 361–374 Search PubMed.
- Z. Korade and A. K. Kenworthy, Neuropharmacology, 2008, 55, 1265–1273 Search PubMed.
- I. Levental and H. Wang, J. Lipid Res., 2020, 61(5), 592–594 Search PubMed.
- F. A. Heberle, M. Doktorova, H. L. Scott, A. Skinkle, M. N. Waxham and I. Levental, PNAS, 2020, 117, 19943–19952 Search PubMed.
- G. Li, Q. Wang, S. Kakuda and E. London, J. Lipid Res., 2020, 61, 758–766 Search PubMed.
- J. K. Sheavly, J. A. Pedersen and R. C. Van Lehn, Nanoscale, 2019, 11, 2767–2778 Search PubMed.
- P. U. Atukorale, Y.-S. Yang, A. Bekdemir, R. P. Carney, P. J. Silva, N. Watson, F. Stellacci and D. J. Irvine, Nanoscale, 2015, 11420–11432 Search PubMed.
- X. Chen, P. Tieleman and Q. Liang, Nanoscale, 2018, 2481–2491 Search PubMed.
- X. Lin, X. Lin and N. Gu, Nanoscale, 2020, 12, 4101–4109 Search PubMed.
- A. Bhat, L. W. Edwards, X. Fu, D. L. Badman, S. Huo, A. J. Jin and Q. Lu, Appl. Phys. Lett., 2016, 109(26), 263106 Search PubMed.
- A. Tiwari, A. Prince, M. Arakha, S. Jha and M. Saleem, Nanoscale, 2018, 10, 3369–3384 Search PubMed.
- E. S. Melby, A. C. Mensch, S. E. Lohse, D. Hu, G. Orr, C. J. Murphy, R. J. Hamers and J. A. Pedersen, Environ. Sci. Nano, 2016, 3, 45–55 Search PubMed.
- J. Egawa, M. L. Pearn, B. P. Lemkuil, P. M. Patel and B. P. Head, J. Physiol., 2016, 594, 4565–4579 Search PubMed.
- A. Linetti, A. Fratangeli, E. Taverna, P. Valnegri, M. Francolini, V. Cappello, M. Matteoli, M. Passafaro and P. Rosa, J. Cell Sci., 2010, 123, 595–605 Search PubMed.
- R. Oropesa-Nuñez, S. Seghezza, S. Dante, A. Diaspro, R. Cascella, C. Cecchi, M. Stefani, F. Chiti and C. Canale, Oncotarget, 2016, 7, 44991–45004 Search PubMed.
- G. van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112–124 Search PubMed.
- N. Puff, C. Watanabe, M. Seigneuret, M. I. Angelova and G. Staneva, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 2105–2114 Search PubMed.
- S. L. Veatch and S. L. Keller, Biophys. J., 2003, 85, 3074–3083 Search PubMed.
- M. Leri, R. Oropesa-Nuñez, C. Canale, S. Raimondi, S. Giorgetti, E. Bruzzone, V. Bellotti, M. Stefani and M. Bucciantini, Biochim. Biophys. Acta, Gen. Subj., 2018, 1862, 1432–1442 Search PubMed.
- M. Leri, F. Bemporad, R. Oropesa-Nuñez, C. Canale, M. Calamai, D. Nosi, M. Ramazzotti, S. Giorgetti, F. S. Pavone, V. Bellotti, M. Stefani and M. Bucciantini, J. Cell. Mol. Med., 2016, 20, 1443–1456 Search PubMed.
- S. J. Marrink, H. J. Risselada, S. Yefimov, D. P. Tieleman and A. H. de Vries, J. Phys. Chem. B, 2007, 111, 7812–7824 Search PubMed.
- F. Simonelli, D. Bochicchio, R. Ferrando and G. Rossi, J. Phys. Chem. Lett., 2015, 6, 3175–3179 Search PubMed.
- R. C. Van Lehn, M. Ricci, P. H. J. Silva, P. Andreozzi, J. Reguera, K. Voïtchovsky, F. Stellacci and A. Alexander-Katz, Nat. Commun., 2014, 5, 4482 Search PubMed.
- M. A. Tahir, R. C. Van Lehn, S. H. Choi and A. Alexander-Katz, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 1207–1215 Search PubMed.
- R. S. Gunderson and A. R. Honerkamp-Smith, Biochim. Biophys. Acta, Biomembr., 2018, 1860, 1965–1971 Search PubMed.
- P. U. Atukorale, Z. P. Guven, A. Bekdemir, R. P. Carney, R. C. Van Lehn, D. S. Yun, P. H. Jacob Silva, D. Demurtas, Y. S. Yang, A. Alexander-Katz, F. Stellacci and D. J. Irvine, Bioconjugate Chem., 2018, 29, 1131–1140 Search PubMed.
- N. Fricke and R. Dimova, Biophys. J., 2016, 111, 1935–1945 Search PubMed.
- P. F. F. Almeida, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 72–85 Search PubMed.
- J. Barnoud, G. Rossi and L. Monticelli, Phys. Rev. Lett., 2014, 112, 068102 Search PubMed.
- J. Barnoud, G. Rossi, S. J. Marrink and L. Monticelli, PLoS Comput. Biol., 2014, 10(10), e1003873 Search PubMed.
- Z. P. Guven, P. H. J. Silva, Z. Luo, U. B. Cendrowska, M. Gasbarri, S. T. Jones and F. Stellacci, J. Visualized Exp., 2019, e58872 Search PubMed.
- E. Reimhult, M. Zäch, F. Höök and B. Kasemo, Langmuir, 2006, 22, 3313–3319 Search PubMed.
- S. J. Marrink, H. J. Risselada, S. Yefimov, D. P. Tieleman and A. H. de Vries, J. Phys. Chem. B, 2007, 111, 7812–7824 Search PubMed.
- D. H. De Jong, S. Baoukina, H. I. Ingólfsson and S. J. Marrink, Comput. Phys. Commun., 2015, 199, 1–7 Search PubMed.
- R. Dasgupta, M. S. Miettinen, N. Fricke, R. Lipowsky and R. Dimova, PNAS, 2018, 115(22), 5756–5761 Search PubMed.
- C. A. López, Z. Sovova, F. J. Van Eerden, A. H. De Vries and S. J. Marrink, J. Chem. Theory Comput., 2013, 9, 1694–1708 Search PubMed.
- R. X. Gu, H. I. Ingólfsson, A. H. De Vries, S. J. Marrink and D. P. Tieleman, J. Phys. Chem. B, 2017, 121, 3262–3275 Search PubMed.
- M. N. Melo, H. I. Ingólfsson and S. J. Marrink, J. Chem. Phys., 2015, 143, 243152 Search PubMed.
- T. A. Wassenaar, H. I. Ingólfsson, R. A. Bockmann, D. P. Tieleman and S. J. Marrink, J. Chem. Theory Comput., 2015, 11, 2144–2155 Search PubMed.
- M. J. Abraham, T. Murtola, R. Schulz, S. Páll, J. C. Smith, B. Hess and E. Lindah, SoftwareX, 2015, 1–2, 19–25 Search PubMed.
- G. Bussi, D. Donadio and M. Parrinello, J. Chem. Phys., 2007, 126, 014101 Search PubMed.
- H. J. C. Berendsen, J. P. M. Postma, W. F. Van Gunsteren, A. DiNola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684 Search PubMed.
- M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182 Search PubMed.
- N. Michaud-Agrawal, E. J. Denning, T. B. Woolf and O. Beckstein, J. Comput. Chem., 2011, 32, 2319–2327 Search PubMed.
- R. Gowers, M. Linke, J. Barnoud, T. Reddy, M. Melo, S. Seyler, J. Domański, D. Dotson, S. Buchoux, I. Kenney and O. Beckstein, in Proceedings of the 15th Python in Science Conference, ed. S. Benthall and S. Rostrup, 2016, pp. 98–105 Search PubMed.
- J. S. Hub, B. L. de Groot and D. Van Der Spoel, J. Chem. Theory Comput., 2010, 6, 3713–3720 Search PubMed.
- W. F. Zeno, A. Rystov, D. Y. Sasaki, S. H. Risbud and M. L. Longo, Langmuir, 2016, 32, 4688–4697 Search PubMed.
- C. S. Scheve, P. A. Gonzales, N. Momin and J. C. Stachowiak, J. Am. Chem. Soc., 2013, 135, 1185–1188 Search PubMed.
- C. S. Ho, N. K. Khadka, F. She, J. Cai and J. Pan, Langmuir, 2016, 32, 6730–6738 Search PubMed.
- L. V. Schäfer, D. H. de Jong, A. Holt, A. J. Rzepiela, A. H. de Vries, B. Poolman, J. A. Killian and S. J. Marrink, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1343–1348 Search PubMed.
- E. Hassan-Zadeh, F. Hussain and J. Huang, Langmuir, 2017, 33, 3324–3332 Search PubMed.
- S. Katira, K. K. Mandadapu, S. Vaikuntanathan, B. Smit and D. Chandler, eLife, 2016, 5, e13150 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nr05366j |
| This journal is © The Royal Society of Chemistry 2020 |