Recent advancement in biomedical applications on the surface of two-dimensional materials: from biosensing to tissue engineering
Abstract
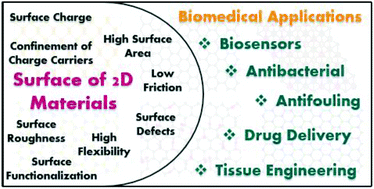
As biosensors and biomedical devices have become increasingly important to everyday diagnostics and monitoring, there are tremendous, and constant efforts towards developing and improving the reliability and versatility of such technology. As they offer high surface area-to-volume ratios and a diverse range of properties, from electronic to optical, two dimensional (2D) materials have proven to be very promising candidates for biological applications and technologies. Due to the dimensionality, 2D materials facilitate many interfacial phenomena that have shown to significantly improve the performance of biosensors, while recent advances in synthesis techniques and surface engineering methods also enable the realization of future biomedical devices. This short review aims to highlight the influence of 2D material surfaces and the properties that arise due to their 2D structure. Using recent (within the last few years) examples of biosensors and biomedical applications, we emphasize the important role of 2D materials in advancing developments and research for biosensing and healthcare.
- This article is part of the themed collections: Editor’s Choice: Recent breakthroughs in nanobiotechnology, Chemistry of 2D materials: graphene and beyond and Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...