Selectivity of nitrate and chloride ions in microporous carbons: the role of anisotropic hydration and applied potentials†
Abstract
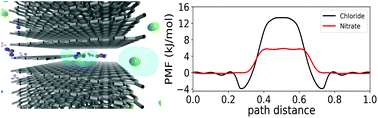
Understanding ion transport in porous carbons is critical for a wide range of technologies, including supercapacitors and capacitive deionization for water desalination, yet many details remain poorly understood. For instance, an atomistic understanding of how ion selectivity is influenced by the molecular shape of ions, morphology of the micropores and applied voltages is largely lacking. In this work, we combined molecular dynamics simulations with enhanced sampling methods to elucidate the mechanism of nitrate and chloride selectivity in subnanometer graphene slit-pores. We show that nitrate is preferentially adsorbed over chloride in the slit-like micropores. This preferential adsorption was found to stem from the weaker hydration energy and unique anisotropy of the ion solvation of nitrate. Beside the effects of ion dehydration, we found that applied potential plays an important role in determining the ion selectivity, leading to a lower selectivity of nitrate over chloride at a high applied potential. We conclude that the measured ion selectivity results from a complex interplay between voltage, confinement, and specific ion effects-including ion shape and local hydration structure.


 Please wait while we load your content...
Please wait while we load your content...