In situ fabrication of MS@MnO2 hybrid as nanozymes for enhancing ROS-mediated breast cancer therapy†
Abstract
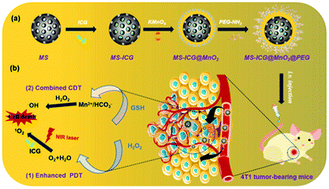
The reactive oxygen species (ROS)-mediated anti-cancer therapy that shows the advantages of tumor specificity, high curative effect, and less toxic side-effects has powerful potential for cancer treatment. However, hypoxia in the tumor microenvironment (TME) and low penetrability of photosensitizers further limit their clinical application. Here, we present a composite core–shell-structured nanozyme (MS-ICG@MnO2@PEG) that consists of a mesoporous silica nanoparticle (MS) core and a MnO2 shell loaded with the photosensitizer indocyanine green (ICG) and then coated with PEG as the photodynamic/chemodynamic therapeutic agent for the ROS-mediated cancer treatment. On the one hand, MS-ICG@MnO2@PEG catalyzes H2O2 to produce O2 for enhanced photodynamic therapy (PDT), and on the other hand, it consumes GSH to trigger a Fenton-like reaction that generates *OH, thus enhancing the chemodynamic therapy (CDT). At the cellular level, MS-ICG@MnO2@PEG nanozymes exhibit good biocompatibility and induce the production of ROS in 4T1 tumor cells. It disrupts the redox balance in tumor cells affecting the mitochondrial function, and specifically kills the tumor cells. In vivo, the MS-ICG@MnO2@PEG nanozymes selectively accumulate at tumor sites and inhibit tumor growth and metastasis in 4T1 tumor-bearing mice. Accordingly, this study shows that the core–shell nanozymes can serve as an effective platform for the ROS-mediated breast cancer treatment by enhancing the combination of PDT and CDT.
- This article is part of the themed collections: Editor’s Choice: Recent breakthroughs in nanobiotechnology and 2020 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...