Open Access Article
Open Access ArticleRealising the electrochemical stability of graphene: scalable synthesis of an ultra-durable platinum catalyst for the oxygen reduction reaction†
Gyen Ming A.
Angel
 a,
Noramalina
Mansor
a,
Rhodri
Jervis
a,
Noramalina
Mansor
a,
Rhodri
Jervis
 a,
Zahra
Rana
a,
Chris
Gibbs
a,
Andrew
Seel
b,
Alexander F. R.
Kilpatrick
a,
Zahra
Rana
a,
Chris
Gibbs
a,
Andrew
Seel
b,
Alexander F. R.
Kilpatrick
 c,
Paul R.
Shearing
c,
Paul R.
Shearing
 a,
Christopher A.
Howard
a,
Christopher A.
Howard
 b,
Dan J. L.
Brett
b,
Dan J. L.
Brett
 *a and
Patrick L.
Cullen
*a and
Patrick L.
Cullen
 *d
*d
aElectrochemical Innovation Lab, Department of Chemical Engineering, University College London, Torrington Place, London WC1E 7JE, UK. E-mail: d.brett@ucl.ac.uk
bDepartment of Physics & Astronomy, University College London, London, WC1E 6BT, UK
cInstitut für Chemie, Humboldt Universität zu Berlin, Brook-Taylor-Straße 2, 12489, Berlin, Germany
dSchool of Engineering and Materials Science (SEMS) and Material Research Institute, Queen Mary University of London, London, E1 4NS, UK. E-mail: p.cullen@qmul.ac.uk
First published on 29th June 2020
Abstract
Creating effective and stable catalyst nanoparticle-coated electrodes that can withstand extensive cycling is a current roadblock in realising the potential of polymer electrolyte membrane fuel cells. Graphene has been proposed as an ideal electrode support material due to its corrosion resistance, high surface area and high conductivity. However, to date, graphene-based electrodes suffer from high defect concentrations and non-uniform nanoparticle coverage that negatively affects performance; moreover, production methods are difficult to scale. Herein we describe a scalable synthesis for Pt nanoparticle-coated graphene whereby PtCl2 is reduced directly by negatively charged single layer graphene sheets in solution. The resultant nanoparticles are of optimal dimensions and can be uniformly dispersed, yielding high catalytic activity, remarkable stability, and showing a much smaller decrease in electrochemical surface area compared with an optimised commercial catalyst over 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The stability is rationalised by identical location TEM which shows minimal nanoparticle agglomeration and no nanoparticle detachment.
000 cycles. The stability is rationalised by identical location TEM which shows minimal nanoparticle agglomeration and no nanoparticle detachment.
1. Introduction
Satisfying global energy demands without damaging the environment is one of the great modern challenges. Using hydrogen in fuel cells provides a route to convert chemical into electrical energy with water as the only by-product at the point of use. Hydrogen fuel cells therefore provide a route to an efficient, environmentally-friendly, non-toxic electrochemical power source suitable for a wide range of applications.1Of the many designs of fuel cells, the hydrogen fuelled polymer electrolyte membrane/proton exchange membrane (PEM) fuel cell is one of the most suitable for transport applications due to its fast start-up time, low temperature operation, solid electrolyte and favourable power-to-weight ratio. Currently, the most widely used catalyst for both the hydrogen oxidation reaction (HOR) at the anode and oxygen reduction reaction (ORR) at the cathode is platinum, or an alloy containing platinum.1,2 The electrochemically active surface area per gram of metal can be maximised by creating platinum nanoparticles with optimized surface area-to-volume ratio. The ideal nanoparticle diameter for a fuel cell cathode is on the order of ∼2 nm.3 Smaller Pt nanoparticles possess more strain, leading to weaker O2 binding and better ORR performance.2 However, below 2 nm the higher proportion of low coordination number Pt atoms which bond strongly with O2 leads to less catalytically active platinum,3,4 moreover they readily agglomerate.5–7
In order to maximize Pt utilization, nanoparticles are typically decorated onto a porous carbon based support; this also promotes electronic connection and allows reactant gases to reach the catalyst. Catalyst durability is a significant challenge, especially when operating at low loading. Platinum nanoparticles degrade via various mechanisms across the 0.6–1 VRHE operating potential range, with carbon corrosion becoming more significant between 1–1.6 VRHE.4 Both of these potential ranges are directly relevant to fuel cell applications, where 0.6–1 VRHE is typical of standard operating voltages, and 1–1.6 VRHE reflects the start-up and shut-down of the fuel cell.8–10
Graphene11 has properties that could provide a high performance catalyst support12 as it has high conductivity13 and a very high surface area. Additionally, graphene has shown chemical resistance in acidic conditions.14 However, the graphene used in the experiments which confirmed these properties was made by the non-scalable micromechanical cleavage or “scotch-tape” method. In order to exploit the properties of graphene on a larger scale much research effort has been dedicated to developing methods for the mass production of graphene.15,16
Liquid phase exfoliation of graphite provides a scalable graphene production method, and the liquids can then be used to efficiently cast the dispersed graphene sheets into working electrodes.16 For producing a graphene-supported catalyst, by far the most common approach is via liquid-processable graphene oxide (GO) created from graphite using a modified Hummer's method.17–26 High temperatures and/or aggressive chemicals are used to only partially reduce the non-conductive GO to conductive reduced graphene oxide rGO.17–26 Finally, the rGO is then modified with the addition of metal nanoparticles. Unfortunately, the multiple energy intensive steps typically include ultracentrifugation and high temperatures, which are difficult to scale. Furthermore, the rGO contains a high number of defects which negatively impacts the electrode's durability,9 especially at higher voltages associated with fuel cell start-up and shut-down (1–1.6 VRHE). As a result, accelerated stress tests are typically only carried out across the 0.6–1 VRHE range, and rarely for more than a few thousand cycles to mitigate the damage that occurs over long cycles and higher loads. Table S1† presents a literature summary of accelerated stress test protocols and results. These generally fall far from the 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycle target set by the US DoE,8,9,17 and rarely consider the 1–1.6 VRHE potential range.
000 cycle target set by the US DoE,8,9,17 and rarely consider the 1–1.6 VRHE potential range.
An alternative strategy to exfoliate layered materials is to intercalate arrays of alkali metals between their layers and then dissolve the resulting compound in polar aprotic solvents.27–30 These solutions can form spontaneously (i.e. without any agitation) with solutes comprising negatively charged 2D materials (termed graphenide in the case for negatively charged graphene) and positively charged cations. Graphenide solutions have been synthesised29–32 using stage 1 graphite intercalation compounds (GICs), which have alkali metals between each graphene layer.33 GICs such as KC8 are known as precursors for metal-graphites34 and it has been shown that the negative charge on the graphenide can be used to reduce some metal salts to form metal particles (Mn, Zn, Cu) on a 10–200 nm scale.35 More recently this has also been achieved for metal and metal oxide nanoparticles.32,36,37
In this work, for the first time, uniformly-sized Pt nanoparticles have been grown on a low-defect graphene support in a scalable, one-pot synthesis. This is achieved by reacting graphenide solutions with PtCl2. It has been shown that using a low and controlled alkali metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) graphene ratio, produces stable, extremely well dispersed Pt nanoparticles of ideal dimensions for PEM fuel cell catalysis. The graphenide-derived platinum nanoparticle/graphene hybrid (GD-Pt/G) is shown to catalyse ORR, with a performance that matches that of a state-of-the-art commercial Pt/C catalyst. Furthermore, GD-Pt/G exhibits excellent durability compared to the commercial Pt/C catalyst across both 0.6–1 VRHE and 1–1.6 VRHE potential range accelerated stress tests. The accelerated stress tests are in line with DoE protocols and are conducted over many more cycles than comparable literature (see Table S1†).
graphene ratio, produces stable, extremely well dispersed Pt nanoparticles of ideal dimensions for PEM fuel cell catalysis. The graphenide-derived platinum nanoparticle/graphene hybrid (GD-Pt/G) is shown to catalyse ORR, with a performance that matches that of a state-of-the-art commercial Pt/C catalyst. Furthermore, GD-Pt/G exhibits excellent durability compared to the commercial Pt/C catalyst across both 0.6–1 VRHE and 1–1.6 VRHE potential range accelerated stress tests. The accelerated stress tests are in line with DoE protocols and are conducted over many more cycles than comparable literature (see Table S1†).
2. Experimental section
2.1 Production of charged graphene dispersion
Graphite (∼325 mesh, Sigma) was evacuated down to a pressure of 10−6 mbar and outgassed at 400 °C until base pressure was achieved. This was then loaded into an argon glove box (H2O < 0.1 ppm, O2 < 0.1 ppm) and placed in a sealed reaction vessel with potassium metal (Sigma, 99.95%) at a K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio of 1
C ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24. This reaction vessel was then placed onto a high integrity gas manifold and evacuated to 10−6 mbar. Anhydrous NH3 was condensed onto the reaction vessel at −60 °C until the characteristic deep-blue colour38 of the dilute (<1 moles percent metal, i.e. <1 moles K for 99 moles NH3) metal-ammonia solution was seen. When the liquid turned colourless, indicating that intercalation had been completed, the NH3 was cryopumped from the sample, leaving a light blue powder39 of KC24(NH3)1.3 graphite intercalation compound (GIC). Previous C13 NMR studies of these GICs have shown that there is only one C atom environment, as expected from an unfunctionalised eclipsed stacking arrangement of the C layers with electrons donated from the metal to the graphite, delocalised.40 This metal-ammonia GIC was then transferred to a glove box where THF was added at a concentration of 5 mg ml−1. In order to increase the concentration of the negatively charged graphene platelets in a timely manner, bath sonication was applied, in a method previously reported.29 The resultant solutions were allowed to settle in the glove box for a further 24 hours before the supernatant was separated from undispersed powder for use.
24. This reaction vessel was then placed onto a high integrity gas manifold and evacuated to 10−6 mbar. Anhydrous NH3 was condensed onto the reaction vessel at −60 °C until the characteristic deep-blue colour38 of the dilute (<1 moles percent metal, i.e. <1 moles K for 99 moles NH3) metal-ammonia solution was seen. When the liquid turned colourless, indicating that intercalation had been completed, the NH3 was cryopumped from the sample, leaving a light blue powder39 of KC24(NH3)1.3 graphite intercalation compound (GIC). Previous C13 NMR studies of these GICs have shown that there is only one C atom environment, as expected from an unfunctionalised eclipsed stacking arrangement of the C layers with electrons donated from the metal to the graphite, delocalised.40 This metal-ammonia GIC was then transferred to a glove box where THF was added at a concentration of 5 mg ml−1. In order to increase the concentration of the negatively charged graphene platelets in a timely manner, bath sonication was applied, in a method previously reported.29 The resultant solutions were allowed to settle in the glove box for a further 24 hours before the supernatant was separated from undispersed powder for use.
2.2 Production of PtCl2 dispersion
PtCl2 was added to THF inside the glove box at a concentration of 1 mg ml−1. This was then sonicated for 30 minutes, keeping the temperature below 30 °C.2.3 Reaction of graphene dispersion with PtCl2
In the glovebox, the PtCl2 dispersion was added to the charged graphene liquid in a stoichiometric amount, assuming a 100% yield of GIC to graphenide production and a reaction where 2 moles of potassium are required to reduce 1 mole of PtCl2. As undispersed GIC powder remained in the bottle the supernatant was taken from, this results in an excess of PtCl2. This was left to react under gentle stirring overnight.2.4 TEM
Once the GD-Pt/G hybrid was synthesized in THF, it was drop-cast onto a holey carbon grid. For identical location TEM, gold mesh “finder” grids were used. TEM was then performed on a Jeol JEM 2100 equipped with a LaB6 source.2.5 Rotating disk electrochemistry
A 40 μl aliquot of GD-Pt/G ink was dropped onto a polished (Al2O3 micro-polish, Bueler) 0.1963 cm2 glassy carbon electrode, achieving a loading of ∼10 μgPt cm−2. Subsequently 8 μl of 0.02 wt% Nafion ionomer solution was added to the surface of the electrode, serving as a binder. This was used with a standard three-electrode cell, containing 0.1 M HClO4 electrolyte solution, a reversible hydrogen electrode (Gaskatel) and a Pt mesh counter electrode. For the commercial Pt/C (Johnson Matthey HiSpec4000), 1 mg of the catalyst was dispersed in 1 ml of 0.05 wt% Nafion in 75%/25% water/IPA mixture. A loading of 35 μgPt cm−2 was deposited onto the glassy carbon electrode. The PtCl2 ink control sample was produced by adding PtCl2/THF dispersion to an amount of THF equivalent to the volume of graphenide solution used in the preparation of GD-Pt/G (in total 0.62 mg ml−1). The electrode was then prepared following the same procedure as used for the GD-Pt/G ink, providing a control sample with the same amount of PtCl2 on the electrode, that would have been on the GD-Pt/G electrode if there had been no reaction with graphenide. Working electrodes were first electrochemically activated via rapid cycling (500 mV s−1, 50 cycles) between 0.05 VRHE and 1.2 VRHE. Cyclic voltammograms were obtained by cycling the working electrode between 0.025 VRHE and 1.2 VRHE at room temperature, under N2 flow, at a scan rate of 20 mV s−1.In order to investigate ORR activity, linear sweep voltammetry was performed at room temperature under constant O2 flow at 1600 rpm. The scans were performed at 20 mV s−1 between −0.01 VRHE and 1 VRHE.
To examine the durability of the platinum nanoparticles and the graphene support, corrosion experiments were carried out across both 0.6–1 VRHE and 1–1.6 VRHE ranges. Here, the working electrode was cycled at 100 mV s−1, and then at various intervals, a full CV was taken between 0.025 VRHE and 1.2 VRHE at 20 mV s−1 in order to obtain the ECSA.41 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles were carried out for each durability test.
000 cycles were carried out for each durability test.
2.6 Image processing
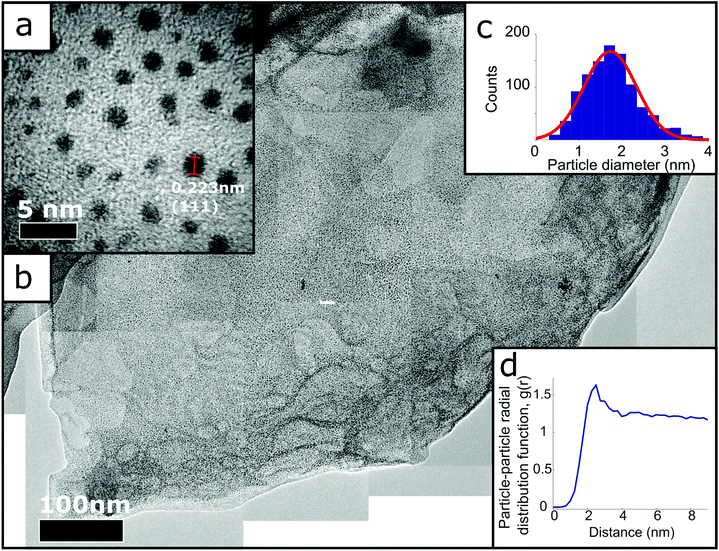
Using Gwyddion software, Fig. 2(b) was thresholded to only select the Pt nanoparticles, making it possible to determine the co-ordinates of every detected nanoparticle centre and measure the distances between each centre. This information was used to create the radial distribution function shown in Fig. 2(d), with the average number density calculated by number of detected particles in an area of known size (754 nanoparticles in 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 nm2).
651 nm2).
3. Results and discussion
The charged graphene dispersion was synthesised via the “metal-ammonia” method, which can produce exclusively single layer graphenide as previously reported29 and is described in detail in the Methods section. The solutions are stable due to the charge on the graphene. The process for making the GD-Pt/G material is shown schematically in Fig. 1. The reaction occurs when platinum(II) chloride dispersed in THF is added to the dispersion of negatively charged graphene sheets in THF. Once the reaction is complete the graphene is uncharged, and as a result the material flocculates,31 with platinum nanoparticles separating the individualised graphene sheets. The restacked structure can be seen in Fig. S1,† where a platinum-covered graphene sheet is disturbed by the transmission electron microscope (TEM) beam. This causes one sheet to slide across another, revealing more of the underlying sheet, which is also decorated with platinum nanoparticles. This is further supported by Raman spectroscopy of GD-Pt/G shown in Fig. S2.†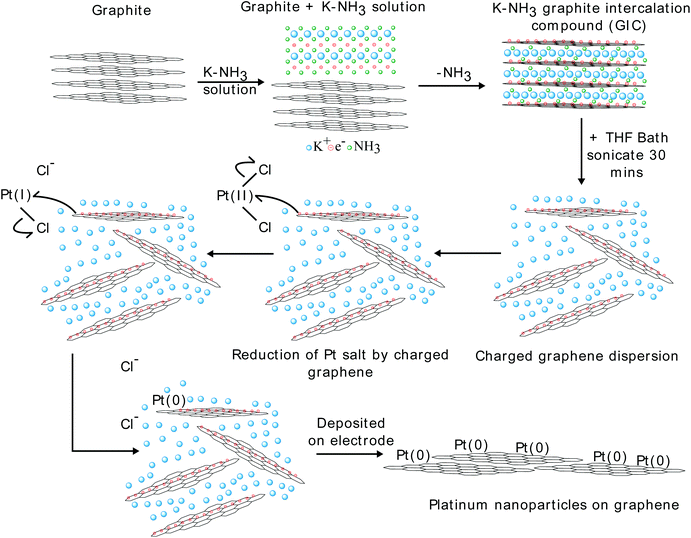 | ||
| Fig. 1 Reaction scheme for the reduction of PtCl2 by graphenide to produce Pt(0) particles on graphene. | ||
The 2D peak can be fitted with a single Lorentzian centred on ca. 2700 cm−1, with a FWHM of ca. 67 cm−1, suggesting a turbostratic restacking of graphene.42 Furthermore, the I(D)/I(G) is 0.33 which is significantly lower than the I(D)/I(G) reported for rGO supports,20,22,43,44 suggesting GD-Pt/G is less defective. Fig. 2 presents TEM micrographs of GD-Pt/G, obtained via the reaction of sonication-aided dissolution of KC24(NH3)1.3 with PtCl2. The resultant material consists of ∼micron-sized layered structures covered uniformly with a high density of nanometre sized objects. Fig. 2(a) shows a close up image of a GD-Pt/G sheet. Analysis of the lattice planes, shown in Fig. S3,† confirms the nanoparticles to be metallic Pt as expected, with the (111) planes of the Pt nanoparticles marked on the micrograph. This suggests that the Pt nanoparticles are formed by the on-sheet reduction of PtCl2 by the delocalised charge present on the graphene sheets.
By manually measuring 1034 nanoparticles in Fig. 2(a), the particle diameter distribution displayed in Fig. 2(c) has been obtained. The histogram is well-fitted by a Gaussian function from which the mean diameter of the Pt was calculated to be (1.7 ± 0.6) nm. By stitching together many nanometre-resolution micrographs (Methods), it was possible to produce a composite TEM micrograph, allowing for the high resolution required to distinguish individual nanoparticles to be realised across a much larger area (ca. 1 μm2). Fig. 2(b) demonstrates the homogeneity of the distribution of the nanoparticles on the graphene surface, with very few examples of agglomerate particles. The distribution of the nanoparticles was investigated by calculating the Pt–Pt site-centre, site-centre radial distribution function, g(r), of the Pt nanoparticles (Fig. 2(d), Methods). The g(r) demonstrates the Pt particles are well ordered with respect to one another. The distribution has a clear peak at 2.5 nm, corresponding to the average inter-particle nearest neighbour distance, and a smaller second peak at 4.75 nm. As well as the size of the particles, the edge-to-edge distance between nanoparticles makes a significant difference in catalytic activity, with a significant increase in specific activity as the separation decreases below 1 nm.45 With an average edge-to-edge distance of 0.8 nm, this proximity effect may contribute to GD-Pt/G's strong electrocatalytic performance (as discussed in the results shown below).
Rotating disk electrode experiments were carried out to investigate the performance of the GD-Pt/G as a catalyst for ORR. Cyclic voltammetry was used to calculate the electrochemical surface area (ECSA) of a GD-Pt/G sample, a commercial Pt/carbon sample and a PtCl2 control sample (Fig. S4†). The hydrogen adsorption and desorption regions of the CV for GD-Pt/G show no distinct peaks, suggesting the broad peaks observed are a convolution of the peaks associated with the crystal facets of platinum and that the nanoparticles are polycrystalline.46 Using X-ray photoelectron spectroscopy (XPS), a typical GD-Pt/G sample was found to be 11.5 wt% platinum, with a corresponding RDE loading weight of 10.3 μgPt cm−2 (Fig. S5†). The ECSA of GD-Pt/G was found to be 94.40 m2 gpt−1, which compares favourably with the ECSA of Pt/carbon, 58.21 m2 gpt−1. The high ECSA can be attributed to the fine distribution of platinum nanoparticles in GD-Pt/G. The PtCl2 control sample, with an ECSA of 0.29 m2 gpt−1, shows that any contribution to excess PtCl2 of catalytic activity, when compared to GD-Pt/G, is negligible (Fig. S4†). Furthermore, there are no features of platinum in the PtCl2 CV, only double layer capacitance.
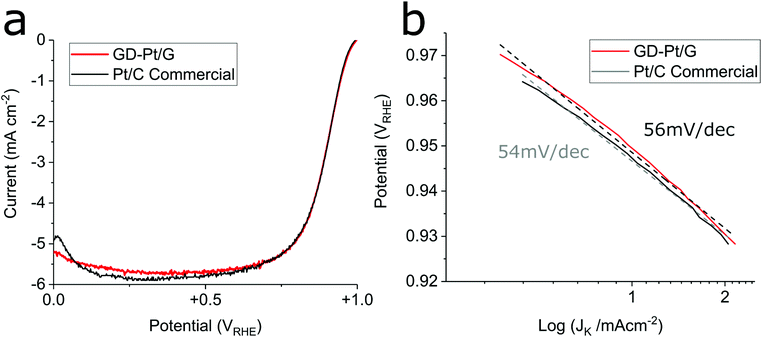
Fig. 3 presents the linear sweep voltammograms and associated Tafel plots obtained for GD-Pt/G and commercial Pt/C electrodes, measured at a rotation rate of 1600 rpm in 0.1 M HClO4, with a scan rate of 20 mV s−1 in accordance to DOE protocols adapted for RDE (See ESI†).
In Fig. 3(a) it can be seen that the GD-Pt/G electrode closely matches the onset and half-wave potentials and limiting current of the highly-optimised commercial Pt/C electrode. Specific and mass activities were calculated from the kinetic current, obtained from the current measured at 0.9 VRHE.41 Although specific activities were found to be similar (465 and 413 μA cmPt−2 for GD-Pt/G and Pt/C respectively), the mass activity of GD-Pt/G was approximately twice as large as of the commercial sample, as shown in Table 1. The observed increased mass activity suggests that the graphene support improves the utilisation of the available platinum nanoparticles, likely due its facilitation of the formation of well-dispersed Pt nanoparticles, and its high conductivity.37,47,48
| Material | ECSA (m2 gpt−1) | Specific activity (μA cmPt−2) | Mass activity (A mgpt−1) |
|---|---|---|---|
| GD-Pt/G | 94.0 | 465 | 0.44 |
| Pt/C | 54.8 | 413 | 0.23 |
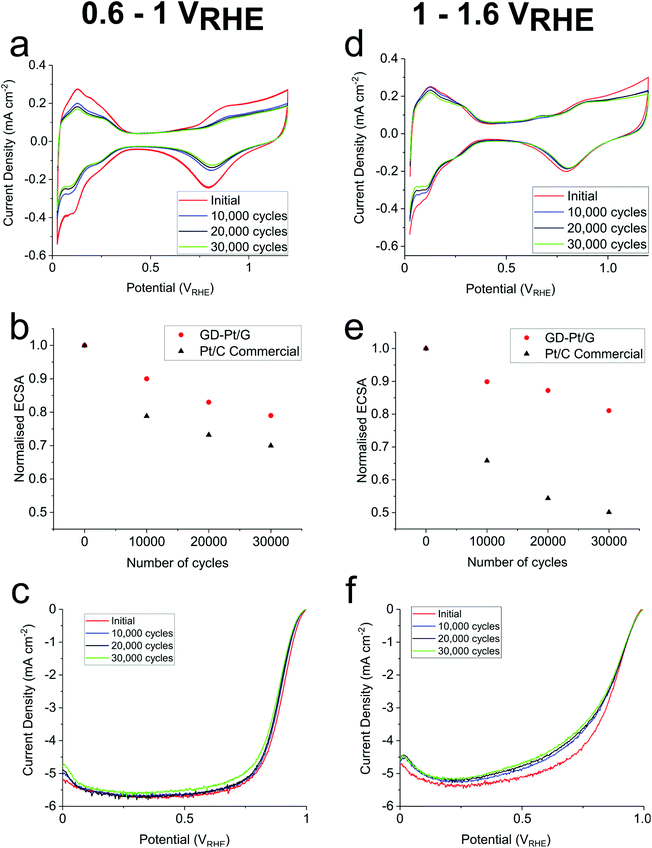
The Tafel plots displayed in Fig. 3(b) were obtained from Fig. 3(a) by calculating mass-transport corrected kinetic current densities and plotting them against potential. The associated Tafel slopes were estimated to be 56 mV dec−1 for GD-Pt/G, and 54 mV dec−1 for commercial Pt/C. These values suggest that for both catalysts, the ORR proceeds via the preferred 4-electron pathway, for which the coverage of the adsorbed oxygen intermediates is the rate limiting factor.1,49,50 The ORR mechanism is further evidenced by rotating ring-disk electrode experiments, in which the number of electrons transferred was found to be above 3.9 across the entirety of the potential range, and the percentage of H2O2 produced remained below 1% (Fig. S6 and S7†). To assess the durability of the GD-Pt/G catalyst, accelerated stress tests were carried out for 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles between both 0.6–1 VRHE and 1–1.6 VRHE based on DoE accelerated stress tests (see ESI†). The cyclic voltammograms, the ORR polarisation curves and the associated relative change in the normalised electrochemical surface areas (ECSAs), are shown in Fig. 4. Fig. 4(a) and (d) show the cyclic voltammograms measured for GD-Pt/G at 10
000 cycles between both 0.6–1 VRHE and 1–1.6 VRHE based on DoE accelerated stress tests (see ESI†). The cyclic voltammograms, the ORR polarisation curves and the associated relative change in the normalised electrochemical surface areas (ECSAs), are shown in Fig. 4. Fig. 4(a) and (d) show the cyclic voltammograms measured for GD-Pt/G at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycle intervals, from which the hydrogen adsorption peaks (0.075–0.4 VRHE) were used to calculate the ECSAs.
000 cycle intervals, from which the hydrogen adsorption peaks (0.075–0.4 VRHE) were used to calculate the ECSAs.
The ECSA values were then normalised to the initial ECSA and plotted against the number of accelerated stress test cycles, presented in Fig. 4(b) and (e). From these figures, it can be seen that, across the 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the ECSA of commercial Pt/C sample degraded by as much as 30% and 50% during the 0.6–1 VRHE and 1–1.6 VRHE, tests respectively. Meanwhile, GD-Pt/G maintained its activity remarkably well, showing a comparatively small ECSA loss of 21% and 19% for the same number of cycles. The stability is further reflected in the ORR polarisation curves shown in Fig. 4(c) and (f), which show negligible change in the onset potentials, and very little change in half-wave potentials and limiting currents after 30
000 cycles, the ECSA of commercial Pt/C sample degraded by as much as 30% and 50% during the 0.6–1 VRHE and 1–1.6 VRHE, tests respectively. Meanwhile, GD-Pt/G maintained its activity remarkably well, showing a comparatively small ECSA loss of 21% and 19% for the same number of cycles. The stability is further reflected in the ORR polarisation curves shown in Fig. 4(c) and (f), which show negligible change in the onset potentials, and very little change in half-wave potentials and limiting currents after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Table S2†).
000 cycles (Table S2†).
These results show our GD-Pt/G material show best in class durability when compared to any platinum graphene based ORR catalyst reported in the literature as summarised in Table S1.† Upon cycling, the decrease in catalytic activity in typical Pt/C catalysts is due to the corrosion of the carbon and the change in distribution of the platinum within the system. Suggested mechanisms for these changes are: size increase of platinum nanoparticles via 3D Ostwald ripening, migration/agglomeration of platinum nanoparticles, platinum dissolution into the electrolyte and the detachment of platinum nanoparticles from the carbon support due to carbon corrosion.5–7
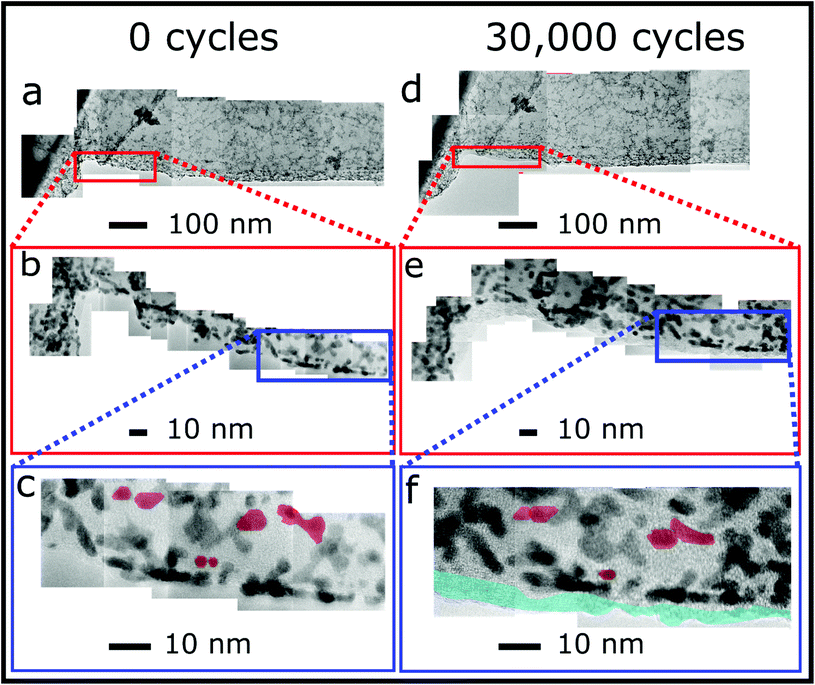
The effects of these mechanisms can be observed using identical location TEM (ILTEM), which offers the unique ability to image the same area of material before and after cycling, where the TEM grid is directly used as the working electrode.7 To further investigate the durability of GD-Pt/G, ILTEM was performed to give a qualitative assessment of which corrosion mechanisms were responsible for activity loss. Gold ILTEM grids were prepared via drop-casting GD-Pt/G ink and examined before and after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 0.6–1 VRHE potential cycles. Fig. 5 shows composite TEM micrograph stitches of the same area of a sample of overlapping sheets of GD-Pt/G at various length scales, before and after the accelerated stress test. Fig. 5(a) and (d) present ca. 1 μm length of material. At this resolution, it can be seen that the GD-Pt/G remains almost completely unchanged, showing clear resistance to the typical corrosion mechanisms seen in commercial Pt/C catalysts.7 The structure of the nanoparticle distribution appears to be unaffected by the cycling: in order to find any differences it is necessary to evaluate the sample at much higher resolutions. The areas shown in the red rectangles in Fig. 5(a) and (d), are shown at a higher resolution in Fig. 5(b) and (e), respectively. The sections in blue are then shown at an even higher resolution in Fig. 5(c) and (f), where the composites are built from atomic resolution micrographs. At this resolution, it is possible to observe small changes in the distribution of the nanoparticles. In Fig. 5(c) and (f), examples of platinum nanoparticles agglomerating have been highlighted in red. As these particles can be seen to have moved small distances to agglomerate, it appears that this has occurred via the migration mechanism rather than via Ostwald ripening. Importantly, there are no distinguishable cases of nanoparticle detachment. The blue area along the edge of the graphene sheet shown in Fig. 5(f) highlights the presence of the Nafion added as part of the accelerated stress test. The changes in platinum nanoparticle distribution and graphene sheets shown in Fig. 5 are much less significant than what is typically observed with commercial Pt/C catalysts,7 where extensive nanoparticle agglomeration and loss of up to half of the nanoparticles can be observed in as few as 4 hours of cycling.51 This is consistent with the small relative decrease in the activity of the GD-Pt/G catalyst. In the majority of methods, nanoparticles are formed via reduction of a platinum precursor by a secondary reducing agent and interact with the supporting material via van der Waals force. Previous studies32,37 have suggested that the distribution of nanoparticles is due to the limited supply of charge available to reduce the platinum precursor. By making use of the metal-ammonia method to intercalate graphite, it has been possible to obtain a stage 1 GIC (KC24(NH3)1.3) in which the graphene sheets have lower charge density than for the typical stage 1 compound KC8.32,37 The lower charge ratio may explain the production of smaller nanoparticles, well-suited for electrocatalysis.
000 0.6–1 VRHE potential cycles. Fig. 5 shows composite TEM micrograph stitches of the same area of a sample of overlapping sheets of GD-Pt/G at various length scales, before and after the accelerated stress test. Fig. 5(a) and (d) present ca. 1 μm length of material. At this resolution, it can be seen that the GD-Pt/G remains almost completely unchanged, showing clear resistance to the typical corrosion mechanisms seen in commercial Pt/C catalysts.7 The structure of the nanoparticle distribution appears to be unaffected by the cycling: in order to find any differences it is necessary to evaluate the sample at much higher resolutions. The areas shown in the red rectangles in Fig. 5(a) and (d), are shown at a higher resolution in Fig. 5(b) and (e), respectively. The sections in blue are then shown at an even higher resolution in Fig. 5(c) and (f), where the composites are built from atomic resolution micrographs. At this resolution, it is possible to observe small changes in the distribution of the nanoparticles. In Fig. 5(c) and (f), examples of platinum nanoparticles agglomerating have been highlighted in red. As these particles can be seen to have moved small distances to agglomerate, it appears that this has occurred via the migration mechanism rather than via Ostwald ripening. Importantly, there are no distinguishable cases of nanoparticle detachment. The blue area along the edge of the graphene sheet shown in Fig. 5(f) highlights the presence of the Nafion added as part of the accelerated stress test. The changes in platinum nanoparticle distribution and graphene sheets shown in Fig. 5 are much less significant than what is typically observed with commercial Pt/C catalysts,7 where extensive nanoparticle agglomeration and loss of up to half of the nanoparticles can be observed in as few as 4 hours of cycling.51 This is consistent with the small relative decrease in the activity of the GD-Pt/G catalyst. In the majority of methods, nanoparticles are formed via reduction of a platinum precursor by a secondary reducing agent and interact with the supporting material via van der Waals force. Previous studies32,37 have suggested that the distribution of nanoparticles is due to the limited supply of charge available to reduce the platinum precursor. By making use of the metal-ammonia method to intercalate graphite, it has been possible to obtain a stage 1 GIC (KC24(NH3)1.3) in which the graphene sheets have lower charge density than for the typical stage 1 compound KC8.32,37 The lower charge ratio may explain the production of smaller nanoparticles, well-suited for electrocatalysis.
The stability of the platinum nanoparticles on GD-Pt/G can be attributed to the direct reduction of the PtCl2 by the negatively charged graphene sheets, which anchors the nanoparticles to them directly, as suggested by Hof et al.32,52 The improved durability of the graphene across the 1–1.6 VRHE accelerated stress test compared with carbon and other reduced graphene oxide supported catalysts is likely due to the method with which the graphene is produced. This results in graphene with a low number of defects, meaning there are fewer sites for the initiation of carbon corrosion.52,53
4. Conclusion
In summary, a scalable method that directly makes use of charged graphene sheets to reduce PtCl2 has been used to produce high quality graphene decorated with well-dispersed platinum nanoparticles (GD-Pt/G). GD-Pt/G has been shown to exhibit excellent activity toward the ORR, closely matching the performance and mechanism of a highly-optimised commercial Pt/C catalyst. Furthermore, GD-Pt/G demonstrates very high stability, showing much smaller relative decreases in electrochemical surface area and performance than commercial Pt/C across a much larger number of cycles than previously reported in the literature. Transmission electron microscopy has been used to characterise the nanoparticle distribution across large surface areas. Using identical location TEM, it has been shown that the relatively small changes in activity post-cycling may be attributed to the excellent durability of the graphene sheets, low levels of agglomeration and lack of detachment of platinum nanoparticles. As a result, it is expected that GD-Pt/G is suitable for scale up for use in membrane electrode assemblies within fuel cells and electrolysers. As a general synthesis method this has the potential to produce different metal nanoparticles finely dispersed across graphene which could be tailored for electrochemical applications across the fields of catalysis, sensors and supercapacitors.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. PLC conceived the project with RJ. PLC and DJB directed the project. TEM was performed by PLC and GMAA. RDE, Raman spectroscopy and accelerated stress tests were performed by GMAA. PLC and GMAA wrote the paper.Conflicts of interest
Some of the authors have filed a patent application based on the preparation of this material (WO/2019/158569). There are no other conflicts of interest.Acknowledgements
PLC would like to thank the EPSRC for funding this project: EP/M506448/1, EP/S001298/1.References
- O. T. Holton and J. W. Stevenson, Platinum Met. Rev., 2013, 57, 259–271 CrossRef.
- S. Guo, S. Zhang and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526–8544 CrossRef CAS PubMed.
- M. Shao, A. Peles and K. Shoemaker, Nano Lett., 2011, 11, 3714–3719 CrossRef CAS PubMed.
- D. Y. Chung, J. M. Yoo and Y. E. Sung, Adv. Mater., 2018, 30, 1704123 CrossRef PubMed.
- Y. Shao, G. Yin and Y. Gao, J. Power Sources, 2007, 171, 558–566 CrossRef CAS.
- L. Dubau, L. Castanheira, G. Berthomé and F. Maillard, Electrochim. Acta, 2013, 110, 273–281 CrossRef CAS.
- M. Arenz and A. Zana, Nano Energy, 2016, 29, 299–313 CrossRef CAS.
- A. Riese, D. Banham, S. Ye and X. Sun, J. Electrochem. Soc., 2015, 162, F783–F788 CrossRef CAS.
- J. Speder, A. Zana, I. Spanos, J. J. K. Kirkensgaard, K. Mortensen, M. Hanzlik and M. Arenz, J. Power Sources, 2014, 261, 14–22 CrossRef CAS.
- H. A. Gasteiger, S. S. Kocha, B. Sompalli and F. T. Wagner, Appl. Catal., B, 2005, 56, 9–35 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- N. M. Julkapli and S. Bagheri, Int. J. Hydrogen Energy, 2015, 40, 948–979 CrossRef CAS.
- M. Liu, R. Zhang and W. Chen, Chem. Rev., 2014, 114, 5117–5160 CrossRef CAS PubMed.
- S. Böhm, Nat. Nanotechnol., 2014, 9, 741–742 CrossRef PubMed.
- L. Banszerus, M. Schmitz, S. Engels, M. Goldsche, K. Watanabe, T. Taniguchi, B. Beschoten and C. Stampfer, Nano Lett., 2016, 16, 1387–1391 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- J. Liu, Q. Ma, Z. Huang, G. Liu and H. Zhang, Adv. Mater., 2019, 1800696, 1800696 CrossRef PubMed.
- R. Kou, Y. Shao, D. Wang, M. H. Engelhard, J. H. Kwak, J. Wang, V. V. Viswanathan, C. Wang, Y. Lin, Y. Wang, I. A. Aksay and J. Liu, Electrochem. Commun., 2009, 11, 954–957 CrossRef CAS.
- H. Yin, H. Tang, D. Wang, Y. Gao and Z. Tang, ACS Nano, 2012, 6, 8288–8297 CrossRef CAS PubMed.
- Y.-X. Huang, J.-F. Xie, X. Zhang, L. Xiong and H.-Q. Yu, ACS Appl. Mater. Interfaces, 2014, 6, 15795–15801 CrossRef CAS PubMed.
- L. Sun, H. Wang, K. Eid, S. M. Alshehri, V. Malgras, Y. Yamauchi and L. Wang, Electrochim. Acta, 2016, 188, 845–851 CrossRef CAS.
- Y. Zheng, S. Zhao, S. Liu, H. Yin, Y. Y. Chen, J. Bao, M. Han and Z. Dai, ACS Appl. Mater. Interfaces, 2015, 7, 5347–5357 CrossRef CAS PubMed.
- S. Xu and P. Wu, J. Mater. Chem. A, 2014, 2, 13682–13690 RSC.
- P. Zhang, W. Tu, R. Wang, S. Cai, J. Wu, Q. Yan, H. Pan, H. Zhang and H. Tang, Int. J. Electrochem. Sci., 2016, 11, 10763–10778 CrossRef CAS.
- X. Mu, Z. Xu, Y. Ma, Y. Xie, H. Mi and J. Ma, Electrochim. Acta, 2017, 253, 171–177 CrossRef CAS.
- M. Wojnicki, M. Luty-Blocho, K. Mech, J. Grzonka, K. Fitzner and K. J. Kurzydlowski, J. Flow Chem., 2015, 5, 22–30 CrossRef CAS.
- C. Vallés, C. Drummond, H. Saadaoui, C. a. Furtado, M. He, O. Roubeau, L. Ortolani, M. Monthioux and A. Pénicaud, J. Am. Chem. Soc., 2008, 1–4 Search PubMed.
- K. Huang, G. Delport, L. Orcin-Chaix, C. Drummond, J. S. Lauret and A. Pénicaud, Nanoscale, 2016, 8, 8810–8818 RSC.
- E. M. Milner, N. T. Skipper, C. A. Howard, M. S. P. Shaffer, D. J. Buckley, K. A. Rahnejat, P. L. Cullen, R. K. Heenan, P. Lindner and R. Schweins, J. Am. Chem. Soc., 2012, 134, 8302–8305 CrossRef CAS PubMed.
- P. L. Cullen, K. M. Cox, M. K. Bin Subhan, L. Picco, O. D. Payton, D. J. Buckley, T. S. Miller, S. A. Hodge, N. T. Skipper, V. Tileli and C. A. Howard, Nat. Chem., 2017, 9, 244–249 CrossRef CAS PubMed.
- A. Catheline, C. Vallés, C. Drummond, L. Ortolani, V. Morandi, M. Marcaccio, M. Iurlo, F. Paolucci and A. Pénicaud, Chem. Commun., 2011, 47, 5470–5472 RSC.
- F. Hof and A. Pénicaud, Chem. – Eur. J., 2018, 24, 16246–16250 CrossRef CAS PubMed.
- M. S. Dresselhaus and G. Dresselhaus, Adv. Phys., 2002, 511, 1–186.
- D. Savioa, C. Trombini and A. Umani-Ronchi, Pure Appl. Chem., 1985, 57, 1887–1896 Search PubMed.
- S. A. Hodge, H. H. Tay, D. B. Anthony, R. Menzel, D. J. Buckley, P. L. Cullen, N. T. Skipper, C. A. Howard and M. S. P. Shaffer, Faraday Discuss., 2014, 172, 311–325 RSC.
- E. G. C. Neiva, V. H. R. Souza, K. Huang, A. Pénicaud and A. J. G. Zarbin, J. Colloid Interface Sci., 2015, 453, 28–35 CrossRef CAS PubMed.
- F. Hof, A. Boni, G. Valenti, K. Huang, F. Paolucci and A. Pénicaud, Chem. – Eur. J., 2017, 23, 15283–15288 CrossRef CAS PubMed.
- M. T. J. H. Lodge, P. Cullen, N. H. Rees, N. Spencer, K. Maeda, J. R. Harmer, M. O. Jones and P. P. Edwards, J. Phys. Chem. B, 2013, 117, 13322–13334 CrossRef CAS PubMed.
- B. R. York and S. A. Solin, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 8206–8220 CrossRef CAS PubMed.
- T. Tsang, R. M. Fronko, H. A. Resing, X. W. Qian and S. A. Solin, Solid State Commun., 1987, 62, 117–120 CrossRef CAS.
- Y. Garsany, O. A. Baturina, K. E. Swider-Lyons and S. S. Kocha, Anal. Chem., 2010, 82, 6321–6328 CrossRef CAS PubMed.
- L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresselhaus, Phys. Rep., 2009, 473, 51–87 CrossRef CAS.
- B. Sravani, P. Raghavendra, Y. Chandrasekhar, Y. Veera Manohara Reddy, R. Sivasubramanian, K. Venkateswarlu, G. Madhavi and L. Subramanyam Sarma, Int. J. Hydrogen Energy, 2020, 45, 7680–7690 CrossRef CAS.
- Y. Kim, D. Lee, Y. Kwon, T. W. Kim, K. Kim and H. J. Kim, J. Electroanal. Chem., 2019, 838, 89–93 CrossRef CAS.
- M. Nesselberger, M. Roefzaad, R. F. Hamou, P. U. Biedermann, F. F. Schweinberger, S. Kunz, K. Schloegl, G. K. H. Wiberg, S. Ashton, U. Heiz, K. J. J. Mayrhofer and M. Arenz, Nat. Mater., 2013, 12, 919–924 CrossRef CAS PubMed.
- K. Yamamoto, D. M. Kolb, R. Kötz and G. Lehmpfuhl, J. Electroanal. Chem., 1979, 96, 233–239 CrossRef.
- E. Antolini, Appl. Catal., B, 2012, 123–124, 52–68 CrossRef CAS.
- F. Hof, M. Liu, G. Valenti, E. Picheau, F. Paolucci and A. Pénicaud, J. Phys. Chem. C, 2019, 123, 20774–20780 CrossRef CAS.
- K. Wang, H. Wang, S. Ji, H. Feng, V. Linkov and R. Wang, RSC Adv., 2013, 3, 12039 RSC.
- T. Shinagawa, A. T. Garcia-esparza and K. Takanabe, Sci. Rep., 2015, 5, 13081 CrossRef PubMed.
- K. J. J. Mayrhofer, J. C. Meier, S. J. Ashton, G. K. H. Wiberg, F. Kraus, M. Hanzlik and M. Arenz, Electrochem. Commun., 2008, 10, 1144–1147 CrossRef CAS.
- E. Bertin, A. Münzer, S. Reichenberger, R. Streubel, T. Vinnay, H. Wiggers, C. Schulz, S. Barcikowski and G. Marzun, Appl. Surf. Sci., 2019, 467–468, 1181–1186 CrossRef CAS.
- Y. Shao, S. Zhang, C. Wang, Z. Nie, J. Liu, Y. Wang and Y. Lin, J. Power Sources, 2010, 195, 4600–4605 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nr03326j |
| This journal is © The Royal Society of Chemistry 2020 |