Anodic hydrazine electrooxidation boosted overall water electrolysis by bifunctional porous nickel phosphide nanotubes on nickel foam†
Abstract
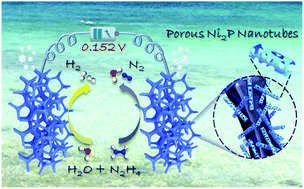
Water electrolysis is an environmentally friendly and sustainable technique for ultra-pure hydrogen production, while expensive electrode materials and high driving voltage have seriously hindered its commercialization process. Here, Earth-abundant bifunctional porous Ni2P hollow nanotubes on nickel foam (Ni2P-HNTs/NF) electrocatalysts are synthesized through a facile self-template method and a phosphating process, which are perfectly combined with the hydrazine electrooxidation reaction (HzOR) boosted water electrolysis. Benefiting from the unique structural characteristic of open-framework and abundant step atoms, Ni2P-HNTs/NF achieves 10 mA cm−2 at 91 mV (vs. RHE) for the cathodic hydrogen evolution reaction and 18 mV (vs. RHE) for the anodic HzOR in a three electrode system, respectively. The corresponding two-electrode hydrazine electrolyzer produces 10 mA cm−2 with a total voltage of only 152 mV for ultra-pure hydrogen production, highlighting a cost-effective and energy-saving water electrolysis mode.


 Please wait while we load your content...
Please wait while we load your content...