Open Access Article
Open Access ArticleCarbon dots-inspired fluorescent cyclodextrins: competitive supramolecular “off–on” (bio)sensors†
Eduardo
De los Reyes-Berbel
 ab,
Inmaculada
Ortiz-Gomez
ab,
Inmaculada
Ortiz-Gomez
 bc,
Mariano
Ortega-Muñoz
bc,
Mariano
Ortega-Muñoz
 ab,
Alfonso
Salinas-Castillo
ab,
Alfonso
Salinas-Castillo
 bc,
Luis Fermin
Capitan-Vallvey
bc,
Luis Fermin
Capitan-Vallvey
 bc,
Fernando
Hernandez-Mateo
bc,
Fernando
Hernandez-Mateo
 ab,
Francisco Javier
Lopez-Jaramillo
ab,
Francisco Javier
Lopez-Jaramillo
 *ab and
Francisco
Santoyo-Gonzalez
*ab and
Francisco
Santoyo-Gonzalez
 *ab
*ab
aDepartment of Organic Chemistry, Biotechnology Institute, Faculty of Sciences, Campus Fuentenueva sn, University of Granada, 18071-Granada, Spain. E-mail: fjljara@ugr.es; fsantoyo@ugr.es
bUnit of Excellence in Chemistry Applied to Biomedicine and the Environment, University of Granada, Spain
cDepartment of Analytical Chemistry, Faculty of Sciences, Campus Fuentenueva sn, University of Granada, 18071-Granada, Spain
First published on 8th April 2020
Abstract
Chromophore-appended cyclodextrins combine the supramolecular loading capabilities of cyclodextrins (CDs) with the optical properties of the affixed chromophores. Among fluorescent materials, carbon dots (CNDs) are attractive and the feasibility of CND-appended CDs as sensors has been demonstrated by different authors. However, CNDs are intrinsically heterogeneous materials and their ulterior functionalization yields hybrid composites that are not well defined in terms of structure and composition. Inspired by the fluorescence properties of 5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (IPCA), the most paradigmatic of the molecular fluorophores detected in CNDs, herein we report two highly efficient synthetic chemical strategies for the preparation of IPCA-appended CDs that behave as CND-based CD “turn off–on” biosensors suitable for the analysis of cholesterol and β-galactosidase activity. We have deconstructed the CND–CD systems to demonstrate that (i) the role of CNDs is limited to acting as a support for the molecular fluorophores produced during their synthesis and (ii) the molecular fluorophores suffice for the determination of the enzymatic activity based on the quenching by p-nitrophenol as a sacrificial quencher.
1. Introduction
Chromophore-appended cyclodextrins (CDs) are spectroscopically active colourful systems engineered to exploit synergistically the supramolecular hosting capabilities of these glucose-based macrocycles with the optical properties of the affixed chromophores.1,2 These dual conjugates are valuable photochemical molecular devices of paramount importance that have found increasing applications in many different areas with a heavy emphasis on imaging, drug delivery, and sensing. Among chromophores, fluorophores provide high sensitivity in analytical applications and a low detection limit in optical imaging methods. Therefore, fluorophore-appended CDs (FCDs) have been profusely investigated as fluorescent chemo- and bio-sensors for detecting a variety of organic and biological compounds.3–6 The principle behind FCDs is the effect of the microenvironment surrounding the chromophore on their fluorescence properties. Based on their fluorescence behaviour, two types of molecular-based FCD sensors, namely “turn-off” and “turn-on” FCDs, have been described.1,6 For the most abundant “turn-off” FCDs, upon complexation of the analyte, a decrease in the fluorescence intensity is observed while for the “turn-on” FCDs an increase in the fluorescence is detected.Fluorophores of diverse nature have been incorporated into FCD-based sensors. Classically, various types of molecular fluorophores (aryl derivatives, dansyls, nitrobenzofurans, xanthenes, cyanines, porphyrins, and phthalocyanines, among others) have been used for CD covalent tagging.6 Molecular-based FCDs are generally constructed using the tools of synthetic chemistry and chemical strategies that ensure isomeric purity and a unitary degree of substitution of native polyhydroxylated CDs since well-characterized systems are required for better performance in most of the sensing applications. Usually, monosubstituted FCDs are synthesized using multistep synthetic procedures that enable the realization of a sole FCD regioisomer.
The outstanding progress made in recent years on nanotechnology has enabled the elaboration of novel CD-modified nanoparticles (NPs). Diverse inorganic NPs (gold, silver, quantum dots and magnetic NPs) and carbonaceous nanomaterials (fullerenes, nanotubes, graphene and carbon dots) provide suitable platforms for the assembly of CDs on their surfaces.7,8 CD-modified NPs combine the supramolecular loading capabilities of CDs with the optical, electronic or magnetic properties of NPs. When these systems are used as fluorescent sensors, NPs behave as chromophores governed by the same principles outlined for their molecular counterparts.
Among fluorescent nanomaterials, metal-free carbon nanodots (CNDs) are leading-edge compounds that have attracted rapidly growing interest because of their outstanding features (cost-efficient and easy preparation, water solubility, low toxicity, biocompatibility, and easy functionalization). CNDs are promising candidates for numerous (bio)applications such as bioimaging, theragnosis, drug delivery and fluorescent (bio)sensing.9–12 Nonetheless, despite the extensive use of native CNDs as (bio)sensors, the reported cases of hybrid CND–CD composites are scarce and limited to their implementation in (bio)analytical applications. The “turn-off” detection of selected (bio)analytes (fullerenes,13 phenolic compounds,14 and enzymes15–17) and, alternatively, more elaborated “turn off–on” systems for the biosensing of steroid compounds and cholesterol18,19 have been described. In the latter case, the competitive hosting between a sacrificial quencher and the desired analyte is used for the successive depletion (“turn-off” state) and restoration (“turn-on” state) of the fluorescence, with beneficial gains in sensitivity.4 From the synthetic point of view, CND–CD composites are usually obtained by the post-synthetic surface modification of already synthesized CNDs. Typically, fluorescent CNDs are treated with a coupling reagent to activate the carboxyl groups on the surface of the CNDs and then reacted with amino-CDs.11,13,15,17,18 However, this strategy is not exempt from drawbacks.
A central issue in CNDs is the origin of the photoluminescence. Although the topic is still a subject of debate, some consensus has been established.20–22 For bottom-up CNDs obtained by the co-pyrolysis of citric acid (CA) and an amine, it is accepted that luminescence primarily results from the molecular state rather than from size differences.23 When α,β-bifunctional ethyleneamines (α,β-diamines, β-amino alcohols or α,β-aminothiols) are used, the formation of highly luminescent molecular fluorophores containing a five-membered fused 2-pyridone skeleton has been reported. The citrazinic derivative 5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (IPCA) is the most paradigmatic molecular fluorophore detected (ESI Scheme S1†).22–30 The plausible mechanism for the formation of IPCA involves, first, the formation of two amide bonds between CA and the amine group of the dopant agent, followed by an intramolecular nucleophilic attack by the β-heteroatom – N, O or S – of the dopant amine to form the five-membered ring fused 2-pyridone. The resulting molecular fluorophores are hypothesized to be located on the surface and/or inside the CNDs. However, as the pyrolysis proceeds, carbon cores are formed with a concomitant consumption of molecular fluorophores, a decrease in the quantum yield and an increase in photostability.23,25,30
Regardless of the top-down or bottom-up strategy used, CNDs are intrinsically heterogeneous materials. Accordingly, the ulterior functionalization of CNDs with diverse compounds, including CDs, yields composites that are not well defined in terms of structure and composition. In order to avoid the drawbacks associated with the heterogeneity of CNDs and inspired by the fluorescence properties of citrazinic acid-based molecular fluorophores, we report herein the preparation of IPCA-appended CDs (IPCA-CDs). Two highly efficient synthetic chemical strategies starting from suitable pre-modified monosubstituted CDs (β- and γ-derivatives) are described. We also demonstrate that these engineered FCDs behave as CND-based CD “turn off–on” biosensors using p-nitrophenol as a quencher. As a proof-of-concept, we validate their capabilities as a non-enzymatic cholesterol biosensor and as a sensor for β-galactosidase activity.
2. Results and discussion
2.1. Chemical synthesis and characterization of IPCA-appended FCDs
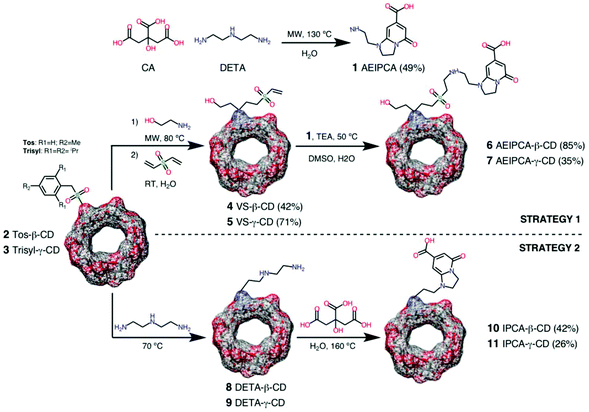
Despite the outstanding optical properties of IPCA derivatives as fluorophores, limited efforts have been devoted to develop synthetic methods that enable their efficient preparation. In the majority of the reported cases, IPCA-based fluorophores are only identified by spectroscopic (NMR and mass) and analytical techniques, and/or isolated in low yields from the prepared CNDs.20,24,25 In order to access the engineered FCDs that avoid the drawback of the heterogeneity of the CNDs as a fluorescent material, we envisaged two different strategies for the synthesis of IPCA-appended CDs that are based on the reaction of CA with a suitable α,β-ethylenediamine molecular motif (Scheme 1).The first approach involves the use of diethylenetriamine (DETA)29,31,32 for the preliminary formation of the IPCA skeleton by a reaction with CA, followed by the covalent grafting of the molecular fluorophore obtained to conveniently functionalized α- and γ-CDs. Thus, in a first step 1-(2-aminoethyl)-5-oxo-1,2,3,5-tetrahydroimidazo[1,2-a]pyridine-7-carboxylic acid (AEIPCA, 1)29,31,32 was prepared using a stand-alone procedure by the microwave-assisted condensation reaction of an equimolecular aqueous mixture of DETA and CA (300 W, 130 °C, 1.5 h). The procedure is novel, it allows the isolation of AEIPCA in a good yield (49%), and spectroscopic characterization correlates well with the already published data.29,31,32AEIPCA was subsequently grafted to mono 6-vinylsulfone-modified CDs (VS-β-CD and VS-γ-CD, 4 and 5) via aza-Michael coupling, exploiting the complementary reactivity of the clickable amine and vinyl sulfone functions.33,34 The efficiency and versatility shown by the Michael-type additions of aminated and thiolated nucleophiles to VS in diverse click metal-free (bio)conjugation scenarios have been previously demonstrated by us.33–35 The synthesis of VS-CDs was carried out with a good yield following the protocol already published by our group.35 It consists of a two-step reaction that involves (a) the microwave-assisted nucleophilic substitution of easily accessible mono 6-O-sulfonyl-CDs (Ts-β-CD and Trisyl-γ-CD, 2 and 336) with ethanolamine to yield the corresponding intermediates, mono-6-(2-hydroxyethyl)amino-CDs, and then (b) vinyl sulfone functionalization of these compounds by the aza-Michael reaction with divinyl sulfone (DVS). The clickable assembly of AEIPCA and VS-CDs (4 and 5) is straightforward, and the corresponding AEIPCA-appended FCDs (6 and 7) were thus isolated.
In an alternative second approach, IPCA was directly synthesized on the CD skeleton using suitable pre-modified monosubstituted α- and γ-CDs containing the α,β-bifunctional ethyleneamine motif. Starting from the same mono 6-O-sulfonyl-CDs (Ts-β-CD and Trisyl-γ-CD, 2 and 336), DETA was first incorporated into the CD scaffold via the substitution reaction of the sulfonyl leaving groups following an already reported procedure for the synthesis of DETA-β-CD (8)37 with minor modifications, and then reacted with CA by a thermal condensation reaction (160 °C, 2 h, under pressure). The desired IPCA-CDs (10 and 11) were thus isolated.
The NMR and mass spectra of IPCA-appended CDs (6, 7, 10 and 11) as well as of all the intermediates (4, 5, 8, and 9) are in accordance with the expected chemical and formula structures (see the ESI†).25,38 UV-visible spectra in water (Fig. S1, ESI†) show two absorption peaks at 235 nm and 355 nm reported for CNDs that are related to the highly fluorescent IPCA.25,30 An additional peak at 285 nm is found in the spectra of compounds 6 and 7 having the sulfone group. As expected, IPCA-appended CDs and compound 1 share the photoluminescence profile of CNDs with a maximum emission peak at 435 nm, typical of CNDs (Fig. S2, ESI†). Regardless of the synthetic approach, the quantum yield (QY) of IPCA-appended CDs (6, 7 and 10, 11) is larger than 0.4, very close to that of compound 1 (QY 0.47) and in the range of that for quinine sulfate (QY 0.55) (Table S1, ESI†). These results demonstrate that the synthesized FCDs have spectroscopic features that resemble those previously reported for CND–CD composites.18 At this point, it is important to recall that a major difference between IPCA-appended CDs and CND–CDs is that the latter are heterogeneous materials, whereas the former are well-defined molecules (Fig. S3, ESI†).
2.2. IPCA-appended FCDs as “off–on” sensors for the quantification of cholesterol (Chol)
As discussed, CND–CDs have been used in (bio)analytical applications, in particular for the biosensing of Chol and steroid compounds, using a “turn off–on” strategy that relies on host–guest recognition,18,19 where the fluorescence is turned off by a sacrificial quencher and recovered (i.e. turned on) upon displacement by an analyte. For these purposes, p-nitrophenol (pNP) has been reported as a valuable quencher, better than o-nitrophenol or m-nitrophenol, and the mechanism has been identified as static quenching.16,19 Additionally, since the absorption spectrum of pNP partially overlaps with the excitation spectrum of the CNDs, the quenching has also been attributed to an inner filter effect.17,39–41Regardless of the mechanism of quenching, the feasibility of using the novel IPCA-appended FCDs as “off–on” sensors depends on the quenching and recovery of the fluorescence by the selective displacement of the quencher by the analyte. Inspired by the works on CND–CDs, we analysed the effect of pNP as a quencher and Chol as an analyte19 on the fluorescence of AEIPCA (1), either alone or in the presence of free β- or γ-CD, and IPCA-appended FCDs (6, 7 and 10, 11). The results (Fig. 1) showed that the fluorescence is quenched by pNP regardless of the presence of CDs, although the extent is larger when the IPCA skeleton is covalently bonded to the CD (compounds 6, 7 and 10, 11). However, at the recovery step only the fluorescence of IPCA-appended FCDs is restored upon the addition of Chol. These results point to major differences in the interaction of pNP with AEIPCA (1) and IPCA-appended FCDs. Data suggest the existence of an unspecific interaction, presumably between the primary amino group of AEIPCA (1) and the phenolate form of pNP, to yield a product that is less fluorescent and not disrupted by Chol. Conversely, the interaction of pNP with IPCA-appended FCDs relies on the formation of an inclusion complex between the CD moiety and pNP, the latter being specifically displaced by Chol. Indeed, in the presence of free β- or γ-CD, Chol leads to a slightly larger quenching of AEIPCA (1) that can be rationalized as the displacement and release of pNP from the inclusion complex with the CD, increasing the amount of pNP available to interact with AEIPCA (1).
The above results on the off/on state of IPCA-appended FCDs resemble those previously described for β-CD-based CND–CDs to detect Chol.19 A more exhaustive analysis of the effect of pNP on the quenching of the fluorescence of IPCA-appended FCDs and the recovery with Chol revealed a linear response with the coefficient of determination R2 = 0.99 (Fig. S4 and S5, ESI†). The time needed to reach the equilibrium for quenching and recovery is 2.5 min and 10 min, respectively (Fig. S6, ESI†), the latter being significantly shorter than the 42 min reported for β-CD-based CND–CDs.19
To investigate the feasibility of quantifying Chol in real samples, FCD 6 was evaluated using human serum as the matrix. The values of the recovery ratio (Table 1) are analogous to those reported for CND–CDs and calf serum as the matrix, indicating that FCD 6 is suitable for the quantification of Chol in real samples.19 All these data support the use of IPCA-appended FCDs as Chol sensors in a similar manner as already established for CND–CDs.
| Sample number | Added Chol (μM) | Sum of added and measured Chol (μM) | Measured Chol (μM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0 | 13.0 | 13.0 ± 1.2 | — |
| 2 | 20 | 33.0 | 36.6 ± 2.1 | 110.9 |
| 3 | 40 | 53.0 | 58.1 ± 1.8 | 109.6 |
| 4 | 60 | 73.0 | 73.5 ± 1.7 | 100.6 |
| 5 | 80 | 93.0 | 99.7 ± 1.3 | 107.2 |
2.3. IPCA-appended FCDs as sensors for the determination of β-galactosidase activity
The ability of pNP to form an inclusion complex with β-CD and quench the fluorescence of β-CD-based CND–CDs has been previously exploited to quantify the activity of β-galactosidase,17 α-glucosidase,42 and alkaline phosphatase.15,17 The assays rely on the quenching of the fluorescence by pNP released upon the action of the enzyme on an analog of the substrate that bears the pNP motif. To further explore the biosensing capabilities of the IPCA-appended FCDs, we decided to investigate these systems as sensors for the determination of β-galactosidase activity. For this purpose, we selected FCD 10. As expected, this compound proved to be a good system to quantify the activity of β-galactosidase from E. coli using p-nitrophenyl β-D-galactopyranoside (pNPG) as a substrate. The results obtained from the kinetics of 8 min show a linear response in the range of 9–280 mU mL−1 and R2 = 0.98 (Fig. S7, ESI†). These values are basically the same as those reported for β-CD-based CND–CDs,15,17 indicating that the role of CNDs in the assay is limited to acting as a support for the molecules responsible for the fluorescence.The facts that the fluorescence of AEIPCA (1), either alone or in the presence of free β- or γ-CD, is quenched by pNP (Fig. 1), and that the activity of α-glucosidase is determined by uncoated CNDs39 suggest that, providing a suitable substrate, AEIPCA (1) may be a simpler system to detect enzymes, in general, and β-galactosidase,17 in particular. To determine the role of β-CD in the quenching of the fluorescence by pNP, the activity of E. coli β-galactosidase was evaluated using AEIPCA (1), either alone or in the presence of free β- or γ-CD, and FCDs 10 and 11, where the fluorophore is covalently bonded to β- and γ-CDs, respectively, as controls. Unexpectedly, at the beginning of the experiment (i.e. t = 0), the fluorescence of FCDs 10 and 11 was 25% and 60% of that for AEIPCA (1), which is not affected by the presence of free CDs (Fig. 2), despite they sharing similar QY (Table S1, ESI†). This result suggests the formation of an inclusion complex between CD and pNPG that is plausible according to theoretical calculations.43 However, quenching is evident only when pNPG and AEIPCA (1) are in close proximity or at a high local concentration, as it is the case for FCDs 10 and 11 due to the covalent bond between the fluorophore and CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pNPG inclusion complex. From the point of view of sensing the enzymatic activity, AEIPCA (1) is as suitable as FCDs 10 and 11 because the values of the enzymatic activity are very close, with the coefficient of variation being 0.15.
pNPG inclusion complex. From the point of view of sensing the enzymatic activity, AEIPCA (1) is as suitable as FCDs 10 and 11 because the values of the enzymatic activity are very close, with the coefficient of variation being 0.15.
3. Conclusions
The feasibility of CD-appended CNDs as sensors has been demonstrated by different authors. These sensors rely on the quenching of the fluorescence of the CNDs by a sacrificial quencher, although the heterogeneity of CNDs hinders their characterization at the molecular level. We have deconstructed the CND–CD systems to demonstrate that (i) the role of CNDs is limited to acting as a support for the molecular fluorophores produced during their synthesis and (ii) the molecular fluorophores suffice for the determination of the enzymatic activity based on the quenching by pNP as a sacrificial quencher. Additionally, we have described two novel routes for the functionalization of CDs with the IPCA molecular fluorophore described as being responsible for the fluorescence of the CNDs. The resulting IPCA-appended FCDs (IPCA-CDs) have been demonstrated to share many of the features of CND–CDs responsible for their use as “off–on” sensors.4. Experimental
4.1. Chemical synthesis
Anhydrous citric acid (CA), cholesterol (Chol), diethylenetriamine (DETA), divinyl sulfone (DVS), ethanolamine, triethylamine (TEA), 4-nitrophenol (pNP), and 4-nitrophenyl β-D-galacto-pyranoside (pNPG) were obtained from Sigma-Aldrich (St Louis, MO, USA). Commercial reagents were used as received without further purification. Mono-6-O-toluensulfonyl-β-cyclodextrin (2) and mono-6-(O-2,4,6-triisopropylbenzenesulfonyl)-γ-cyclodextrin (3)36 were prepared following the already reported procedures. TLC was performed on Merck silica gel 60 F254 aluminium sheets. Flash column chromatography was performed on silica gel (Merck, 230–400 mesh, ASTM). 1H and 13C NMR spectra were recorded at room temperature using a Varian Direct Drive (400, 500, and 600 MHz) spectrometer. Chemical shifts (δ) are given in ppm; J values are given in Hz. MALDI-ToF mass spectra were recorded on an Autoflex Bruker spectrometer using HCCA and NaI as matrices. Optical rotations were recorded using a PerkinElmer 341 polarimeter at room temperature. Fluorescence spectra and UV-Vis spectra were recorded using a Varian Cary Eclipse luminescence spectrometer and a Specord 200 Plus Instrument (Analytik Jena), respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 to 6
0.25 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), yielding pyrone derivative 1 (164 mg, 49%): Mp 204 °C (dec); IR (neat):ν = 1636, 1535, 1509, 1490, 1446, 1411, 1368, 1293, 1255, 1104, 761, 707, 609, 590, 549 cm−1; 1H NMR (D2O, 400 MHz): 6.10 (d, J = 1.2 Hz, 1H), 5.91 (d, J = 1.3 Hz, 1H), 4.14 (t, J = 8.9 Hz, 2H), 3.77 (t, J = 8.9 Hz, 2H), 3.59 (t, J = 6.0 Hz, 4H), 3.32 (t, J = 6.0 Hz, 2H). 13C NMR (D2O, 126 MHz): δ = 172.71, 162.63, 153.38, 152.38, 103.14, 85.03, 47.49, 44.11, 43.66, 36.72; HR-MS (ESI+): m/z = found 224.1026, calcd for C10H14N3O3 [M + H]+: 224.1035.
0.5), yielding pyrone derivative 1 (164 mg, 49%): Mp 204 °C (dec); IR (neat):ν = 1636, 1535, 1509, 1490, 1446, 1411, 1368, 1293, 1255, 1104, 761, 707, 609, 590, 549 cm−1; 1H NMR (D2O, 400 MHz): 6.10 (d, J = 1.2 Hz, 1H), 5.91 (d, J = 1.3 Hz, 1H), 4.14 (t, J = 8.9 Hz, 2H), 3.77 (t, J = 8.9 Hz, 2H), 3.59 (t, J = 6.0 Hz, 4H), 3.32 (t, J = 6.0 Hz, 2H). 13C NMR (D2O, 126 MHz): δ = 172.71, 162.63, 153.38, 152.38, 103.14, 85.03, 47.49, 44.11, 43.66, 36.72; HR-MS (ESI+): m/z = found 224.1026, calcd for C10H14N3O3 [M + H]+: 224.1035.
(a) 6-Deoxy-6-(2-hydroxyethyl) ((vinylsulfonyl)methyl)amino-β-cyclodextrin (4) was obtained as a solid (140 mg, 85%).35
(b) 6-Deoxy-6-(2-hydroxyethyl) ((vinylsulfonyl)methyl)amino-γ-cyclodextrin (5) was obtained as a solid (130 mg, 70%): Mp: 235 °C (dec); IR (neat): ν = 3350, 2927, 1738, 1651, 1366, 1154, 1079, 1026, 941, 849, 757, 706, 582, 529 cm−1; [α]D +153.6. (c 0.25, H2O); 1H NMR (500 MHz, DMSO-d6-D2O, selected signals) δ: 6.87 (m, 1H), 6.17 (m, 2H), 5.13–4.75 (several brs, 8H); 13C NMR (126 MHz, DMSO-d6-D2O): δ 137.49, 130.27, 102.28, 101.68, 81.44, 80.92, 73.37, 72.94, 72.81, 72.64, 72.35, 59.47, 51.03, 40.02, 39.85, 39.69, 39.52, 39.35, 39.18, 39.02; HR-MS (MALDI-TOF): m/z = found 1458.4738, calcd for C54H92NO42S [M + H]+: 1458.4815; found 1480.5160, calcd for C54H92NNaO42S [M + Na]+: 1480.4634.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 9 mL) was added triethylamine (50 μL, 0.3 mmol). The reaction mixture was heated at 50 °C for 6 h and then acetone (50 mL) was added. The resulting precipitate was collected by centrifugation and successively washed with acetone (40 mL) and ether (40 mL). The obtained solid was purified by column chromatography to yield the corresponding IPCA-appended FCDs (6 or 7):
2, 9 mL) was added triethylamine (50 μL, 0.3 mmol). The reaction mixture was heated at 50 °C for 6 h and then acetone (50 mL) was added. The resulting precipitate was collected by centrifugation and successively washed with acetone (40 mL) and ether (40 mL). The obtained solid was purified by column chromatography to yield the corresponding IPCA-appended FCDs (6 or 7):
(a) IPCA-β-FCDs (6): Column chromatography (acetonitrile/water/ammonia 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) gave 6 (140 mg, 92%) as a solid: Mp: 192 °C (dec); [α]D +44.8 (c 0.25, H2O); IR (neat): ν = 3214, 1649, 1551, 1412, 1077, 1028, 612 cm−1; 1H NMR (500 MHz, DMSO-d6-D2O, selected signals): δ 5.97 (s, 1H), 5.76 (s, 1H), 4.85 (d, J = 3.2 Hz, 1H), 4.83–4.79 (m, 5H), 4.78 (d, J = 3.4 Hz, 1H), 3.96 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, DMSO-d6): δ 168.09, 160.79, 152.92, 104.34, 102.15, 102.04, 101.97, 101.90, 101.72, 83.82, 81.67, 81.61, 81.54, 81.39, 80.96, 73.17, 73.03, 72.97, 72.93, 72.46, 72.30, 72.19, 72.06, 70.76, 60.09, 59.91, 59.12, 56.10, 54.67, 52.53, 50.17, 47.94, 47.31, 45.76, 45.64, 42.81, 42.19, 40.02, 39.85, 39.69, 39.52, 39.35, 39.19, 39.02; HR-MS (MALDI-TOF): m/z = found 1541.5027, calcd for C58H94N4NaO40S [M + Na]+: 1541.5063; found 1519.4538, calcd for C58H95N4O40S [M + H]+:1519.5243; found 1557.4644, calcd for C58H94N4KO40S [M + K]+: 1557.4802.
0.1) gave 6 (140 mg, 92%) as a solid: Mp: 192 °C (dec); [α]D +44.8 (c 0.25, H2O); IR (neat): ν = 3214, 1649, 1551, 1412, 1077, 1028, 612 cm−1; 1H NMR (500 MHz, DMSO-d6-D2O, selected signals): δ 5.97 (s, 1H), 5.76 (s, 1H), 4.85 (d, J = 3.2 Hz, 1H), 4.83–4.79 (m, 5H), 4.78 (d, J = 3.4 Hz, 1H), 3.96 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, DMSO-d6): δ 168.09, 160.79, 152.92, 104.34, 102.15, 102.04, 101.97, 101.90, 101.72, 83.82, 81.67, 81.61, 81.54, 81.39, 80.96, 73.17, 73.03, 72.97, 72.93, 72.46, 72.30, 72.19, 72.06, 70.76, 60.09, 59.91, 59.12, 56.10, 54.67, 52.53, 50.17, 47.94, 47.31, 45.76, 45.64, 42.81, 42.19, 40.02, 39.85, 39.69, 39.52, 39.35, 39.19, 39.02; HR-MS (MALDI-TOF): m/z = found 1541.5027, calcd for C58H94N4NaO40S [M + Na]+: 1541.5063; found 1519.4538, calcd for C58H95N4O40S [M + H]+:1519.5243; found 1557.4644, calcd for C58H94N4KO40S [M + K]+: 1557.4802.
(b) IPCA-γ-FCDs (7): Column chromatography (acetonitrile/water/ammonia 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) gave 7 (59 mg, 35%) as a solid: Mp: 198 °C (dec); [α]D +76.4 (c 0.25, H2O); IR (neat): ν = 3231, 1736, 1651, 1555, 1417, 1079, 1024, 996, 759, 705, 613, 527 cm−1; 1H NMR (400 MHz, D2O, selected signals): δ 6.19 (s, 1H), 6.03 (s, 1H), 5.20–5.09 (m, 8H); 13C NMR (126 MHz, DMSO-d6-D2O): δ 173.47, 164.09, 154.64, 105.26, 103.29, 103.13, 102.02, 84.30, 82.30, 82.28, 82.22, 82.10, 82.05, 82.02, 80.60, 74.52, 74.49, 74.42, 74.34, 74.28, 73.97, 73.80, 73.70, 73.67, 73.52, 73.48, 73.45, 73.37, 73.29, 73.22, 73.15, 61.67, 61.60, 61.48, 59.26, 57.81, 56.50, 51.68, 51.17, 49.18, 47.99, 46.43, 45.15, 44.88, 42.01, 40.03, 39.86, 39.69, 39.52, 39.35, 39.18, 39.01; HR-MS (MALDI-TOF): m/z = found 1681.5402, calcd for C64H105N4O45S [M + H]+: 1681.5772; found 1703.5564, calcd for C64H104N4NaO45S [M + Na]+: 1703.5591; found 1719.5828, calcd for C64H104KN4O45S [M + K]+: 1719.5330.
0.1) gave 7 (59 mg, 35%) as a solid: Mp: 198 °C (dec); [α]D +76.4 (c 0.25, H2O); IR (neat): ν = 3231, 1736, 1651, 1555, 1417, 1079, 1024, 996, 759, 705, 613, 527 cm−1; 1H NMR (400 MHz, D2O, selected signals): δ 6.19 (s, 1H), 6.03 (s, 1H), 5.20–5.09 (m, 8H); 13C NMR (126 MHz, DMSO-d6-D2O): δ 173.47, 164.09, 154.64, 105.26, 103.29, 103.13, 102.02, 84.30, 82.30, 82.28, 82.22, 82.10, 82.05, 82.02, 80.60, 74.52, 74.49, 74.42, 74.34, 74.28, 73.97, 73.80, 73.70, 73.67, 73.52, 73.48, 73.45, 73.37, 73.29, 73.22, 73.15, 61.67, 61.60, 61.48, 59.26, 57.81, 56.50, 51.68, 51.17, 49.18, 47.99, 46.43, 45.15, 44.88, 42.01, 40.03, 39.86, 39.69, 39.52, 39.35, 39.18, 39.01; HR-MS (MALDI-TOF): m/z = found 1681.5402, calcd for C64H105N4O45S [M + H]+: 1681.5772; found 1703.5564, calcd for C64H104N4NaO45S [M + Na]+: 1703.5591; found 1719.5828, calcd for C64H104KN4O45S [M + K]+: 1719.5330.
DETA-γ-CD (9): Mp: 216 °C (dec); [α]D +125.6 (c 0.25, H2O); IR (neat): ν = 3320, 2925, 1738, 1651, 1366, 1154, 1079, 1025, 940, 847, 757, 705, 581, 527 cm−1; 1H NMR (400 MHz, D2O): δ 5.43 (s, 1H), 5.27–5.05 (m, 7H), 4.07–3.77 (several multiples, 32H), 3.74–3.57 (several m, 16H), 3.47 (m, 2H), 3.01 (m, 2H), 2.92–2.80 (several m, 4H); 13C NMR (101 MHz, D2O) δ 101.73, 101.63, 80.43, 80.17, 73.30, 72.91, 72.27, 71.79, 71.55, 71.18, 69.33, 60.49, 60.25, 47.53, 47.32, 47.24, 38.69; HR-MS (ES+-TOF): m/z = found 1382.5306, calcd for C52H92N3O39 [M + H]+: 1382.5308.
(a) IPCA-β-FCDs (10): Column chromatography (acetonitrile/water/ammonia 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) gave 10 (280 mg, 42%) as a solid: Mp: 228 °C (dec); [α]D +91.6 (c 0.25, H2O); IR (neat): ν = 3214, 1717, 1651, 1553, 1366, 1230, 1151, 1076, 1022, 946, 753, 704, 610, 576, 528 cm−1; 1H NMR (500 MHz, D2O, selected signals): δ 6.15 (s, 1H), 6.00 (s, 1H), 5.15–4.99 (m, 7H), 4.19 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, D2O): δ 175.82, 165.53, 156.26, 155.72, 105.60, 104.55, 104.18, 87.90, 86.14, 84.19, 83.84, 83.73, 83.43, 75.80, 75.67, 75.20, 74.77, 74.56, 74.53, 74.43, 74.39, 63.42, 63.01, 62.88, 62.79, 51.70, 50.21, 48.06, 46.85, 46.47; HR-MS (ES+-TOF): m/z = found 1340.4678, calcd for C52H81N3O37 [M + H]+: 1340.4627.
0.1) gave 10 (280 mg, 42%) as a solid: Mp: 228 °C (dec); [α]D +91.6 (c 0.25, H2O); IR (neat): ν = 3214, 1717, 1651, 1553, 1366, 1230, 1151, 1076, 1022, 946, 753, 704, 610, 576, 528 cm−1; 1H NMR (500 MHz, D2O, selected signals): δ 6.15 (s, 1H), 6.00 (s, 1H), 5.15–4.99 (m, 7H), 4.19 (t, J = 8.7 Hz, 2H); 13C NMR (126 MHz, D2O): δ 175.82, 165.53, 156.26, 155.72, 105.60, 104.55, 104.18, 87.90, 86.14, 84.19, 83.84, 83.73, 83.43, 75.80, 75.67, 75.20, 74.77, 74.56, 74.53, 74.43, 74.39, 63.42, 63.01, 62.88, 62.79, 51.70, 50.21, 48.06, 46.85, 46.47; HR-MS (ES+-TOF): m/z = found 1340.4678, calcd for C52H81N3O37 [M + H]+: 1340.4627.
(b) IPCA-γ-FCDs (11): Column chromatography (acetonitrile/water/ammonia 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) gave 11 (195 mg, 26%) as a solid: Mp: 213 °C (dec); [α]D +70.8 (c 0.25, H2O): IR (neat): ν = 3214, 1646, 1552, 1420, 1078, 1026, 941, 757, 613, 528 cm−1; 1H NMR (500 MHz, DMSO-d6-D2O, selected signals): δ 5.98 (s, 1H), 5.80 (s, 1H), 4.86 (m, 8H); 13C NMR (126 MHz, D2O): δ 172.95, 162.80, 153.30, 152.93, 103.51, 101.73, 101.67, 101.61, 101.57, 101.49, 100.74, 85.16, 82.72, 80.79, 80.50, 80.40, 80.35, 80.29, 79.11, 74.67, 73.29, 72.90, 72.84, 72.71, 72.36, 72.28, 72.24, 72.13, 72.04, 72.01, 71.85, 71.78, 71.71, 71.63, 71.55, 67.32, 66.00, 60.71, 60.27, 60.21, 60.14, 59.92, 48.61, 47.64, 45.13, 44.80, 43.76, 43.20; HR-MS (ESI−): m/z = found 1500.5084, calcd for C58H90N3O42 [M − H]−: 1500.5004, found 749.7476, calcd for C58H89N3O42 [M − 2H]2−: 749.7466.
0.1) gave 11 (195 mg, 26%) as a solid: Mp: 213 °C (dec); [α]D +70.8 (c 0.25, H2O): IR (neat): ν = 3214, 1646, 1552, 1420, 1078, 1026, 941, 757, 613, 528 cm−1; 1H NMR (500 MHz, DMSO-d6-D2O, selected signals): δ 5.98 (s, 1H), 5.80 (s, 1H), 4.86 (m, 8H); 13C NMR (126 MHz, D2O): δ 172.95, 162.80, 153.30, 152.93, 103.51, 101.73, 101.67, 101.61, 101.57, 101.49, 100.74, 85.16, 82.72, 80.79, 80.50, 80.40, 80.35, 80.29, 79.11, 74.67, 73.29, 72.90, 72.84, 72.71, 72.36, 72.28, 72.24, 72.13, 72.04, 72.01, 71.85, 71.78, 71.71, 71.63, 71.55, 67.32, 66.00, 60.71, 60.27, 60.21, 60.14, 59.92, 48.61, 47.64, 45.13, 44.80, 43.76, 43.20; HR-MS (ESI−): m/z = found 1500.5084, calcd for C58H90N3O42 [M − H]−: 1500.5004, found 749.7476, calcd for C58H89N3O42 [M − 2H]2−: 749.7466.
4.2. Fluorescence studies
| QY = QYst (msm/mst)(η2sm/η2st) |
4.3. Fluorimetric assays
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grant no. CTQ2017-86125-P and CTQ2016-78754-C2-1-R (Ministerio de Economía, Industria y Competitividad, Spain).References
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- R. Pinalli, A. Pedrini and E. Dalcanale, Chem. Soc. Rev., 2018, 47, 7006–7026 RSC.
- A. Ueno, Supramol. Sci., 1996, 3, 31–36 CrossRef CAS.
- H. Ikeda, in Artificial Receptors for Chemical Sensors, ed. V. M. Y. Mirsky and A. K. Yatsimirsky, Willey, 2011, ch. 4, pp. 113–114 Search PubMed.
- T. Ogoshi and A. Harada, Sensors, 2008, 8, 4961–4982 CrossRef CAS PubMed.
- G. Benkovics, M. Malanga and E. Fenyvesi, Int. J. Pharm., 2017, 531, 689–700 CrossRef CAS PubMed.
- G. Cutrone, J. M. Casas-Solvas and A. Vargas-Berenguel, Int. J. Pharm., 2017, 531, 621–639 CrossRef CAS PubMed.
- H. Shelley and R. J. Babu, J. Pharm. Sci., 2018, 107, 1741–1753 CrossRef CAS PubMed.
- H. T. Shi, J. F. Wei, L. Qiang, X. Chen and X. W. Meng, J. Biomed. Nanotechnol., 2014, 10, 2677–2699 CrossRef CAS PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- X. Sun and Y. Lei, TrAC, Trends Anal. Chem., 2017, 89, 163–180 CrossRef CAS.
- H. Behboudi, G. Mehdipour, N. Safari, M. Pourmadadi, A. Saei, M. Omidi, L. Tayebi and M. Rahmandoust, in Advanced Structured Materials, 2019, vol. 104, pp. 145–179 Search PubMed.
- A. Cayuela, M. Laura Soriano and M. Valcárcel, Analyst, 2016, 141, 2682–2687 RSC.
- Z.-Y. Lin, Y.-C. Kuo, C.-J. Chang, Y.-S. Lin, T.-C. Chiu and C.-C. Hu, RSC Adv., 2018, 8, 19381–19388 RSC.
- C. Tang, Z. Qian, Y. Huang, J. Xu, H. Ao, M. Zhao, J. Zhou, J. Chen and H. Feng, Biosens. Bioelectron., 2016, 83, 274–280 CrossRef CAS PubMed.
- C. Tang, J. Zhou, Z. S. Qian, Y. Y. Ma, Y. Y. Huang and H. Feng, J. Mater. Chem. B, 2017, 5, 1971–1979 RSC.
- M. Mao, T. Tian, Y. He, Y. Ge, J. Zhou and G. Song, Microchim. Acta, 2018, 185, 1–6 CrossRef CAS PubMed.
- M. Luo, Y. F. Hua, Y. R. Liang, J. J. Han, D. H. Liu, W. T. Zhao and P. Wang, Biosens. Bioelectron., 2017, 98, 195–201 CrossRef CAS PubMed.
- Q. Sun, S. Fang, Y. Fang, Z. Qian and H. Feng, Talanta, 2017, 167, 513–519 CrossRef CAS PubMed.
- D. Qu and Z. Sun, Mater. Chem. Front., 2020, 4, 400–420 RSC.
- M. K. Barman and A. Patra, J. Photochem. Photobiol., C, 2018, 37, 1–22 CrossRef CAS.
- Y. Xiong, J. Schneider, E. V. Ushakova and A. L. Rogach, Nano Today, 2018, 23, 124–139 CrossRef CAS.
- S. J. Zhu, X. H. Zhao, Y. B. Song, S. Y. Lu and B. Yang, Nano Today, 2016, 11, 128–132 CrossRef CAS.
- W. Kasprzyk, S. Bednarz and D. Bogdal, Chem. Commun., 2013, 49, 6445–6447 RSC.
- Y. B. Song, S. J. Zhu, S. T. Zhang, Y. Fu, L. Wang, X. H. Zhao and B. Yang, J. Mater. Chem. C, 2015, 3, 5976–5984 RSC.
- W. Kasprzyk, S. Bednarz, P. Zmudzki, M. Galica and D. Bogdal, RSC Adv., 2015, 5, 34795–34799 RSC.
- J. Zhang, L. Yang, Y. Yuan, J. Jiang and S.-H. Yu, Chem. Mater., 2016, 28, 4367–4374 CrossRef CAS.
- J. Schneider, C. J. Reckmeier, Y. Xiong, M. von Seckendorff, A. S. Susha, P. Kasak and A. L. Rogach, J. Phys. Chem. C, 2017, 121, 2014–2022 CrossRef CAS.
- X. Meng, Y. Wang, X. Liu, M. Wang, Y. Zhan, Y. Liu, W. Zhu, W. Zhang, L. Shi and X. Fang, Opt. Mater., 2018, 77, 48–54 CrossRef CAS.
- M. Shamsipur, A. Barati, A. A. Taherpour and M. Jamshidi, J. Phys. Chem. Lett., 2018, 9, 4189–4198 CrossRef CAS PubMed.
- X. Liu, H.-B. Li, L. Shi, X. Meng, Y. Wang, X. Chen, H. Xu, W. Zhang, X. Fang and T. Ding, J. Mater. Chem. C, 2017, 5, 10302–10312 RSC.
- W. K. Zhang, L. J. Shi, Y. Q. Liu, X. R. Meng, H. Xu, Y. Q. Xu, B. Y. Liu, X. M. Fang, H. B. Li and T. Ding, RSC Adv., 2017, 7, 20345–20353 RSC.
- J. Morales-Sanfrutos, J. Lopez-Jaramillo, M. Ortega-Munoz, A. Megia-Fernandez, F. Perez-Balderas, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Org. Biomol. Chem., 2010, 8, 667–675 RSC.
- F. J. Lopez-Jaramillo, F. Hernandez-Mateo and F. Santoyo-Gonzalez, in Integrative Proteomics, ed. H. C. M. Leung, T.-K. Man and R. J. Flores, IntechOpen, 2012, pp. 301–326 Search PubMed.
- T. del Castillo, J. Morales-Sanfrutos, F. Santoyo-Gonzalez, S. Magez, F. J. Lopez-Jaramillo and J. A. Garcia-Salcedo, ChemMedChem, 2014, 9, 383–389 CrossRef CAS PubMed.
- R. Palin, S. J. A. Grove, A. B. Prosser and M. Q. Zhang, Tetrahedron Lett., 2001, 42, 8897–8899 CrossRef CAS.
- R. J. Jiang, B. Yang, D. Yi, F. Wang, B. Han, Y. L. Zhao, X. L. Liao, J. Yang and C. Z. Gao, J. Polym. Eng., 2014, 34, 133–139 CAS.
- W. Kasprzyk, P. Krzywda, S. Bednarz and D. Bogdal, RSC Adv., 2015, 5, 90473–90477 RSC.
- S. Huang, E. L. Yang, J. D. Yao, Y. Liu and Q. Xiao, Microchim. Acta, 2018, 185, 9333–9342 Search PubMed.
- N. Xiao, S. G. Liu, S. Mo, N. Li, Y. J. Ju, Y. Ling, N. B. Li and H. Q. Luo, Talanta, 2018, 184, 184–192 CrossRef CAS PubMed.
- M. Zheng, Z. Xie, D. Qu, D. Li, P. Du, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CrossRef CAS PubMed.
- S. Y. Liu, H. Wang, T. He, L. Qi and Z. Q. Zhang, Luminescence, 2016, 31, 96–101 CrossRef CAS PubMed.
- S. S. Kiselev and Y. A. Borisov, J. Struct. Chem., 2016, 57, 849–854 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectroscopic characterization of new compounds, and ESI schemes, figures, and tables. See DOI: 10.1039/d0nr01004a |
| This journal is © The Royal Society of Chemistry 2020 |