Comparative evaluation of MAX, MXene, NanoMAX, and NanoMAX-derived-MXene for microwave absorption and Li ion battery anode applications†
Abstract
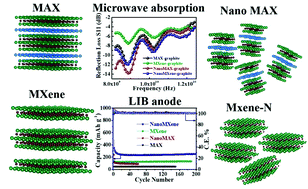
MAX and MXene phases possess unique physical properties, encompassing the realms of both ceramics and metals. Their nanolaminated layered configuration, high anisotropic electrical conductivity, and ability to scatter electromagnetic radiation are beneficial in multiple applications. Herein, detailed applications of MAX and MXene are studied in the fields of microwave absorption and Li ion batteries (LIB). In particular, coatings based on MAX, MXene, ball-milled NanoMAX, and NanoMAX-derived-MXene (MXene-N) and their composites are examined in terms of their comparative efficacy for the aforesaid applications. NanoMAX and MXene-N based composites with graphite exhibit superior performance with specific reflection loss values (representing absorbance when measured with metal-backing) of −21.4 and −19 dB cm3 g−1, respectively, as compared to their bulk counterparts, that too with a low density (0.63 g cm−3) and very small thickness (0.03 mm). These performance improvements in absorbance in only 30 μm coatings can be attributed to reflective losses compounded with multiple internal reflections within the nanocomposite intensified by dielectric losses, arising from high interface density. The pristine samples were also studied for their performance as Li ion battery anodes. Herein, MXene-N exhibits the best performance with a specific capacity of 330 mA h g−1 at 100 mA g−1 and excellent cycling stability tested up to 1000 cycles.
- This article is part of the themed collection: Editor’s Choice: Controlling anisotropy in nanomaterials


 Please wait while we load your content...
Please wait while we load your content...