Dexamethasone loaded bilayered 3D tubular scaffold reduces restenosis at the anastomotic site of tracheal replacement: in vitro and in vivo assessments†
Abstract
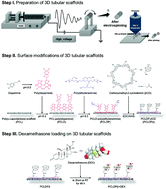
Despite recent developments in the tracheal tissue engineering field, the creation of a patient specific substitute possessing both appropriate mechanical and biointerfacial properties remains challenging. Most tracheal replacement therapies fail due to restenosis at the anastomosis site. In this study, we designed a robust, biodegradable, 3D tubular scaffold by combining electrospinning (ELSP) and 3D (three-dimensional) printing techniques for use in transplantation therapy. After that, we loaded dexamethasone (DEX) onto the 3D tubular scaffold using mild surface modification reactions by using polydopamine (PDA), polyethyleneimine (PEI), and carboxymethyl–β-cyclodextrin (βCD). As a result, the fabricated 3D tubular scaffold had robust mechanical properties and the chemical modifications were confirmed to have proceeded successfully by physico-chemical analysis. The surface treatments allowed for a larger amount of DEX to be loaded onto the βCD modified scaffold as compared to the bare group. In vitro and in vivo studies demonstrated that the DEX loaded 3D tubular scaffold exhibited significantly enhanced anti-inflammation activity, enhanced tracheal mucosal regeneration, and formation of a patent airway. From our results, we believe that our system may represent an innovative paradigm in tracheal tissue engineering by providing proper mechanical properties and successful formation of tracheal tissue as a means of remodeling and healing tracheal defects for use in transplantation therapy.


 Please wait while we load your content...
Please wait while we load your content...