Continuous ultrathin UiO-66-NH2 coatings on a polymeric substrate synthesized by a layer-by-layer method: a kind of promising membrane for oil–water separation†
Abstract
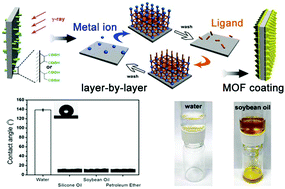
In this paper, we successfully controllably synthesize continuous nanothickness MOF coatings (NTMCs) by a layer-by-layer method on a polymeric substrate. The polymeric substrate was pretreated with high energy γ-irradiation to induce a high surface density of living reactive groups, which ensure the formation of continuous surface-integrated NTMCs. SEM, FT-IR spectroscopy and XPS were used to characterize NTMCs. The thickness and morphology were tuned by the LBL cycles, and NTMCs with a thickness of ∼44 nm were obtained. The chemical bonds between the NTMCs and polymeric substrate were confirmed by XPS and EDS. Moreover, the NTMCs exhibit good performance for oil–water separation. We believe that our work will promote the design and precise synthesis of high-performance MOF based membranes for multiple practical applications in future.


 Please wait while we load your content...
Please wait while we load your content...