Exfoliation of metal–organic frameworks into efficient single-layer metal–organic nanosheet electrocatalysts by the synergistic action of host–guest interactions and sonication†
Abstract
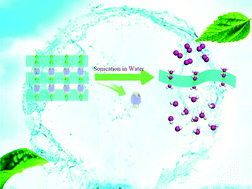
Single-layer two-dimensional metal–organic framework (MOF) nanosheets combine the characteristic of highly ordered structures of MOFs and unique physical and chemical properties of two-dimensional (2D) materials, which is beneficial for developing high-performance electrocatalysts and studying the structure–property relationships. However, the instability of coordination bonds during exfoliation results in great difficulties in their preparation and characterization. This work takes advantage of the anisotropy of coordination bonds in three-dimensional (3D) pillared-layer MOFs and selectively breaks the interlayer bonding through substitution of the pillared ligands by capping solvent molecules synergized with sonication in a solvent over a relatively short time course (30 min), thus leading to single-layer metal–organic nanosheets, which retain the 2D layered structure of the original crystalline counterpart. The as-synthesized single-layer metal–organic nanosheets are efficient electrocatalysts for the oxygen evolution reaction (OER) with a turnover frequency as high as 0.144 s−1 and 0.294 s−1 at overpotentials of 400 mV and 500 mV in neutral electrolyte media, respectively, which are higher than other heterogeneous catalysts.
- This article is part of the themed collection: Editor’s Choice: Controlling anisotropy in nanomaterials


 Please wait while we load your content...
Please wait while we load your content...