Open Access Article
Open Access ArticlePredictive theoretical screening of phase stability for chemical order and disorder in quaternary 312 and 413 MAX phases†
Martin
Dahlqvist
 * and
Johanna
Rosen
*
* and
Johanna
Rosen
*
Thin Film Physics, Department of Physics, Chemistry and Biology (IFM), Linköping University, SE-581 83 Linköping, Sweden. E-mail: martin.dahlqvist@liu.se; johanna.rosen@liu.se
First published on 4th December 2019
Abstract
In this work we systematically explore a class of atomically laminated materials, Mn+1AXn (MAX) phases upon alloying between two transition metals, M′ and M′′, from groups III to VI (Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W). The materials investigated focus on so called o-MAX phases with out-of-plane chemical ordering of M′ and M′′, and their disordered counterparts, for A = Al and X = C. Through use of predictive phase stability calculations, we confirm all experimentally known phases to date, and also suggest a range of stable ordered and disordered hypothetical elemental combinations. Ordered o-MAX is favoured when (i) M′ next to the Al-layer does not form a corresponding binary rock-salt MC structure, (ii) the size difference between M′ and M′′ is small, and (iii) the difference in electronegativity between M′ and Al is large. Preference for chemical disorder is favoured when the size and electronegativity of M′ and M′′ is similar, in combination with a minor difference in electronegativity of M′ and Al. We also propose guidelines to use in the search for novel o-MAX; to combine M′ from group 6 (Cr, Mo, W) with M′′ from groups 3 to 5 (Sc only for 312, Ti, Zr, Hf, V, Nb, Ta). Correspondingly, we suggest formation of disordered MAX phases by combing M′ and M′′ within groups 3 to 5 (Sc, Ti, Zr, Hf, V, Nb, Ta). The addition of novel elemental combinations in MAX phases, and in turn in their potential two-dimensional MXene derivatives, allow for property tuning of functional materials.
Introduction
In the 1960s a family of atomically laminated ternary carbide and nitride materials was discovered.1,2 In the 1990s, these so-called MAX phases gained renewed attention when shown to combine metallic and ceramic attributes like high electric and thermal conductivity, machinability, oxidation resistance, and high temperature mechanical properties.3,4 The MAX phases are of the general formula Mn+1AXn (n = 1–3), where Mn+1Xn sheets, based on a transition metal M and X as C or N, are interleaved by one atom thick A-layers (A is an A-group element). A short notion typically used to describe MAX phases are 211, 312, and 413, depending on the value of n being 1, 2, and 3, respectively. MAX phases are important as parent materials for their two-dimensional (2D) derivative, MXene, realized from selective etching of the A-element.5,6 MXenes show promise for a wide range of applications, including energy storage and electromagnetic interference shielding.7,8A common route for property tuning is through alloying, which for MAX phases can be realized with a fourth element on either M, A, or X site, as demonstrated to change e.g. hardness,9 to tune properties for isotropic thermal expansion,10 and to introduce magnetic characteristics,11 Traditionally, MAX phase alloys have to a large extent been disordered solid solutions with challenges in attaining an exact a priori composition.12 However, in 2014 Liu et al. synthesized a chemically ordered 312 MAX phase, Cr2TiAlC2,13 defined by out-of-plane ordering through alternating M-layers based on one M-element only, later coined o-MAX. This was soon followed by other out-of-plane ordered phases, Mo2TiAlC2,14,15 Mo2ScAlC2,16 Cr1.5V1.5AlC2,17 and corresponding 413 in the form of Mo2Ti2AlC3 and Cr2V2AlC3.15,17 Common for o-MAX phases are the two crystallographic different M-sites, with Wyckoff positions 4f (M′) and 2a (M′′) for 312 and 4f (M′) and 4e (M′′) for 413. This is opposed to the most recent discovery of a family of in-plane ordered 211 MAX phase related materials, coined i-MAX, where the two metals have a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio within each metal layer.18–23
1 ratio within each metal layer.18–23
Chemically ordered o-MAX and i-MAX phases are highly interesting as they allow for incorporation of non-traditional MAX phase elements, e.g., Sc,16,18,23 Y,19,21,23 and W,22 but also for the improved control of the alloy composition. However, there has been no extensive systematic study in the quest for yet hypothetical ordered MAX phases other than for Ti-based o-MAX.24 In this work we focus on alloying between two metals, M′ and M′′, in quaternary 312 and 413 o-MAX phases, to systematically explore chemically ordered (out-of-plane) and disordered distributions of M′ and M′′ with M elements from groups 3 to 6 (Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) while keeping A and X equal to Al and C, respectively. The A = Al choice is motivated by Al being prone to chemical etching for MXene derivation, as shown for the o-MAX phases Cr2TiAlC2, Mo2TiAlC2, Mo2ScAlC2, and Mo2Ti2AlC3.16,25
This study on o-MAX phases is structured into two parts, starting with predictive stability calculations for chemically ordered and disordered distributions of M′ and M′′. We confirm the stability of the quaternary phases reported to date, and also suggest several ordered as well as disordered novel alloys yet to be experimentally discovered. In the second part, we investigate the impact of choice of element M′ and M′′, and effect of size and electronegativity of M′, M′′ and Al, for the formation of chemically ordered (o-MAX) or disordered MAX phases. We also propose guidelines for which elemental combinations to use in search for novel o-MAX phases, by combining M′ and M′′ from specific groups, and how to promote chemical order vs. disorder.
Method
All first-principles calculations were performed by means of density functional theory (DFT) and the projector augmented wave method,26,27 as implemented within the Vienna ab initio simulation package (VASP) version 5.4.1.28–30 We used the non-spin polarized generalized gradient approximation (GGA) as parameterized by Perdew–Burke–Ernzerhof (PBE) for treating the electron exchange and correlation effects.31 For Cr-based phases we used the spin-polarized version with multiple spin configurations considered within the unit cell in line with ref. 24. Presented results are for the lowest energy spin configuration. We also used a plane-wave energy cut-off of 400 eV, and the Monkhorst–Pack scheme for sampling of the Brillouin zone.32 The total energy is minimized through relaxation of the unit-cell shape and volume, and internal atomic positions.In this work we assume the o-MAX chemical order with the notation of M′ facing the Al-layer, while M′′ is being sandwiched between carbon layers in the 312 and 413 MAX phase structures. For 312 this corresponds to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition of M′
2 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C with M′ at 4f and M′′ at 2a Wyckoff positions, and for the 413 this corresponds to a 2
C with M′ at 4f and M′′ at 2a Wyckoff positions, and for the 413 this corresponds to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composition of M′
3 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C with M′ at 4f and M′′ at 4e Wyckoff positions. In addition, for the 312 MAX phase, five semi-ordered structures have been modelled, with a 2
C with M′ at 4f and M′′ at 4e Wyckoff positions. In addition, for the 312 MAX phase, five semi-ordered structures have been modelled, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition of M′
2 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, as shown in Fig. 1a. For the 413 MAX phase, a total of 22 unique ordered structures have been modelled within the unit cell, with a 2
C, as shown in Fig. 1a. For the 413 MAX phase, a total of 22 unique ordered structures have been modelled within the unit cell, with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composition of M′
3 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, as shown in Fig. S1.† Four selected configurations are shown in Fig. 1b. Note that the o-MAX structure has previously been shown to be dynamically stable.33,34 In the present work we considered M′ and M′′ from groups 3 to 6; Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W. To model chemical disorder of M′ and M′′ on the M sublattices we used the special quasi-random structure (SQS) method35 with supercell sizes of 4 × 4 × 1 unit cells, i.e. 192 and 256 atoms for the 312 and 413 MAX phases, respectively. Convergency tests show that the supercells used give a qualitatively accurate representation and a quantitative convergency in terms of calculated formation enthalpies, equilibrium volumes, and lattice parameters.
C, as shown in Fig. S1.† Four selected configurations are shown in Fig. 1b. Note that the o-MAX structure has previously been shown to be dynamically stable.33,34 In the present work we considered M′ and M′′ from groups 3 to 6; Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W. To model chemical disorder of M′ and M′′ on the M sublattices we used the special quasi-random structure (SQS) method35 with supercell sizes of 4 × 4 × 1 unit cells, i.e. 192 and 256 atoms for the 312 and 413 MAX phases, respectively. Convergency tests show that the supercells used give a qualitatively accurate representation and a quantitative convergency in terms of calculated formation enthalpies, equilibrium volumes, and lattice parameters.
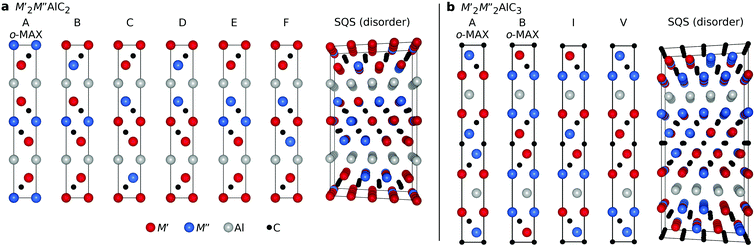 | ||
Fig. 1 Schematic illustration of considered chemical order of M′ and M′′ for (a) a 312 MAX phase structure with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition of M′ 2 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′ M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and (b) a 413 MAX phase structure with a 2 C and (b) a 413 MAX phase structure with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composition of M′ 3 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′ M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, where M′, M′′, Al, and C atoms are represented in red, blue, grey, and black, respectively. In (b), only selected structures are shown for the 413 MAX phase, for further details see Fig. S1.† C, where M′, M′′, Al, and C atoms are represented in red, blue, grey, and black, respectively. In (b), only selected structures are shown for the 413 MAX phase, for further details see Fig. S1.† | ||
The thermodynamic stability of quaternary MAX phases is investigated at 0 K with respect to decomposition into any combination of competing phases. The most competing set of competing phases, denoted equilibrium simplex, is identified using a linear optimization procedure36,37 which have been proven successful to confirm already experimentally known MAX phases as well as predicting the existence of new ones.11,37–41 The stability of the quaternary MAX phase is quantified in terms of formation enthalpy ΔHcp by comparing its energy to the energy of the equilibrium simplex according to
| ΔHcp = E(MAX) − E(equilibrium simplex). | (1) |
A phase is concluded stable when ΔHcp < 0. Here E(MAX) represent the chemically ordered or disordered MAX phase structure of lowest energy. However, when T ≠ 0 K, the contribution from configurational entropy due to disorder of M′ and M′′ on the M sublattices will decrease the Gibbs free energy ΔGdisordercp as approximated by
| ΔGdisordercp[T] = ΔHdisordercp − TΔS, | (2) |
ΔS = −2kB[z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(z) + (1 − z)ln(1 − z)], ln(z) + (1 − z)ln(1 − z)], | (3) |
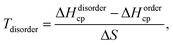 | (4) |
Results and discussion
Stability of ternary 312 and 413 MAX phases
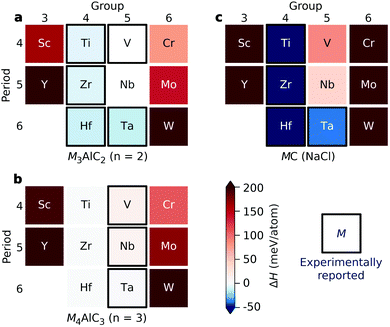
We start by investigating the thermodynamic stability of the ternary M3AlC2 and M4AlC3 MAX phases. Fig. 2 shows the calculated formation enthalpy ΔHcp at 0 K for both M3AlC2 (n = 2) and M4AlC3 (n = 3), with corresponding identified equilibrium simplex listed in Tables S1 and S2.† Thermodynamically stable, or close to stable, MAX phases with n = 2 and 3 are found for M from groups 4 and 5, while MAX phases with M from groups 3 and 6 are far from stable. Experimentally known phases are all identified as stable or close to stable (Hcp < +12 meV per atom). In addition, Nb3AlC2 and M4AlC3 (M = Ti, Zr, Hf) are also predicted to be stable MAX phases (ΔHcp < 0) but are yet to be experimentally discovered. Note that all four of these stable hypothetical MAX phases are competing with the formation of other known MAX phases, as indicated in Tables S1 and S2.†The trends in thermodynamic stability for both M3AlC2 and M4AlC3 is similar to the trends found for the binary MC phase in its rock salt structure (see Fig. 2c), where M from groups 4 (Ti, Zr, Hf) and 5 (V, Nb, Ta) is found to be stable or close to stable while M from groups 3 (Sc, Y) and 6 (Cr, Mo, W) is far from stable (ΔHcp > +200 meV per atom). The comparable trends can be related to the stacking of M and C in the Mn+1Cn layers of the MAX phases which is also found along the 111 direction of rock salt MC.
Order and disorder in quaternary 312 MAX phases
For each 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition of M′
2 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (M′ and M′′ = Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) we solve the linear optimization problem and identify the equilibrium simplex, see Table S3,† and its corresponding ΔHcp and Tdisorder. Fig. 3 shows ΔHcp for different chemical ordering; six ordered (A to F, depicted in Fig. 1a) and one disordered (SQS). The spread in ΔHcp among the considered chemical order configurations is rather small for some systems, e.g. Ti–M′′ and V–M′′, while others show much larger variation, e.g. Cr–M′′ and Mo–M′′.
C (M′ and M′′ = Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) we solve the linear optimization problem and identify the equilibrium simplex, see Table S3,† and its corresponding ΔHcp and Tdisorder. Fig. 3 shows ΔHcp for different chemical ordering; six ordered (A to F, depicted in Fig. 1a) and one disordered (SQS). The spread in ΔHcp among the considered chemical order configurations is rather small for some systems, e.g. Ti–M′′ and V–M′′, while others show much larger variation, e.g. Cr–M′′ and Mo–M′′.
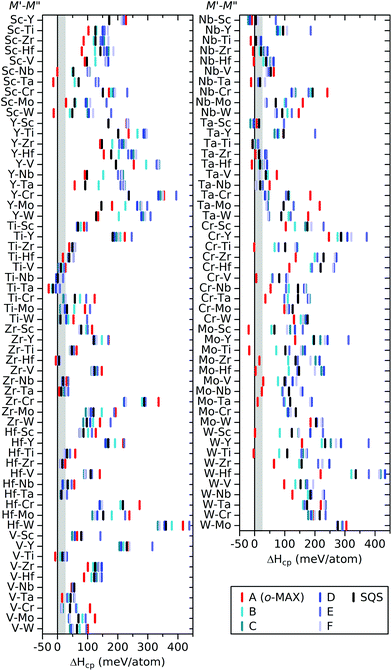 | ||
Fig. 3 Calculated formation enthalpy ΔHcp of the 312 MAX phase structures with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition of M′ 2 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′ M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, for six chemically ordered (A to F, as shown in Fig. 1a) and one chemically disordered (SQS) distribution of M′ and M′′. The grey area represents 0 ≤ ΔHcp ≤ 25 and includes those phases which are close to stable. C, for six chemically ordered (A to F, as shown in Fig. 1a) and one chemically disordered (SQS) distribution of M′ and M′′. The grey area represents 0 ≤ ΔHcp ≤ 25 and includes those phases which are close to stable. | ||
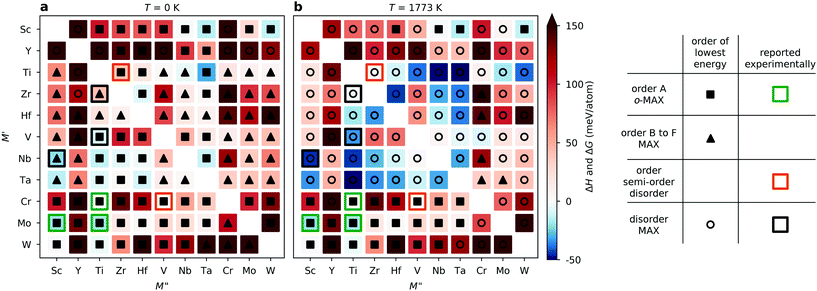
The long list in Fig. 3 of ΔHcp for 110 compositions represented with different configurations of chemical order does not provide a clear picture of whether chemical order or disorder are to be preferred at typical synthesis conditions. In Fig. 4, we therefore visualize the trends in thermodynamic stability using a heatmap, where M′ and M′′ are sorted according to the periodic group in which the metal lies. The background colour represents the calculated thermodynamic stability for chemical order of lowest energy, with a blue region representing stable phases (ΔHcp or ΔGcp < 0). We also use a symbol representation to denote the type of chemical order of lowest energy. In addition, experimentally known MAX phases are marked by a square, where the colour represent the type of reported chemical order. Fig. 4a depicts the calculated formation enthalpy at 0 K, showing that the vast majority of M′ and M′′ combinations are chemically ordered, with 55 o-MAX (filled squares) and 45 of order B to F (filled triangles). Reported o-MAX phases to date are identified as stable; Cr2TiAlC2,13 Mo2ScAl2,16 and Mo2TiAlC2.14 However, the result obtained at 0 K do not capture the MAX phases reported with disorder between M′ and M′′; (Zr0.67Ti0.33)3AlC2,42 (V0.67Ti0.33)3AlC2,43 and (Nb0.67Sc0.33)3AlC2.44
Since typical bulk synthesis of MAX phase are performed around 1773 K we need to consider the contribution from configurational entropy to the Gibbs free energy, using eqn (2) and (3) for structures with disordered distribution of M′ and M′′ (SQS). Fig. 4b depicts either the calculated formation enthalpy ΔHcp (for ordered phases) or Gibbs free energy of formation ΔGcp at 1773 K (for disordered phases), depending on which order is of lowest energy. In such a representation, 79 out of 110 M′ and M′′ combinations are identified as disordered, and out of these 28 are stable, including the MAX phases reported to date with disorder on M-sublattices. Furthermore, 31 phases are identified as o-MAX, with 7 being stable, including those reported experimentally; Cr2TiAlC2,13 Mo2ScAl2,16 and Mo2TiAlC2.14 For Cr2VAlC2, we find the o-MAX to be of lowest energy, with ΔHcp = 6 meV per atom, and it has indeed been synthesized, albeit with some intermixing of V on the Cr site (M′), (Cr0.75V0.25)2VAlC2.17 For M′ = Ti and M′′ = Zr, both Ti2ZrAlC2 o-MAX and disordered (Ti0.67Zr0.33)3AlC2 have been reported.42,45 Our theoretical results indicate that disorder is preferred, though a more in-depth discussion of these discrepancies will be presented below.
Beyond the phases reported to date, we predict four stable o-MAX phases; Sc2NbAlC2, Sc2TaAlC2, Sc2WAlC2, and W2TiAlC2. An additional six o-MAX phases are close to stable; Mo2M′′AlC2 (M′′ = Zr, Hf, V, Nb, Ta) and W2ScAlC2. We also identify 26 systems which are stable, though with a preference for disorder at T < 1773 K, with most of these phases combining M′ and M′′ from groups 4 and 5.
Order and disorder in 413 MAX phases
Identification of the equilibrium simplex was made for each 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composition of M′
3 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′
M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C (M′ and M′′ = Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W), see Table S3.† The formation enthalpy ΔHcp, calculated using eqn (1), is shown in Fig. 5 for different chemical ordering; two ordered o-MAX (A and B), order of type I and V (see Fig. 1b), an additional 18 ordered structures (other), and one disordered (SQS). A schematic illustration of the 22 ordered structures is shown in Fig. S1.† Note that B is the inverse of A, and hence doublets are found, e.g., Mo2Ti2AlC3 of type A is equal to Ti2Mo2AlC3 of type B. The spread in ΔHcp between the considered configurations is more pronounced than for to the 312 systems, but shows a similar overall trend with less spread in energies for certain systems, e.g. Ti–M′′, while others show a much larger spread, e.g. Mo–M′′.
C (M′ and M′′ = Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W), see Table S3.† The formation enthalpy ΔHcp, calculated using eqn (1), is shown in Fig. 5 for different chemical ordering; two ordered o-MAX (A and B), order of type I and V (see Fig. 1b), an additional 18 ordered structures (other), and one disordered (SQS). A schematic illustration of the 22 ordered structures is shown in Fig. S1.† Note that B is the inverse of A, and hence doublets are found, e.g., Mo2Ti2AlC3 of type A is equal to Ti2Mo2AlC3 of type B. The spread in ΔHcp between the considered configurations is more pronounced than for to the 312 systems, but shows a similar overall trend with less spread in energies for certain systems, e.g. Ti–M′′, while others show a much larger spread, e.g. Mo–M′′.
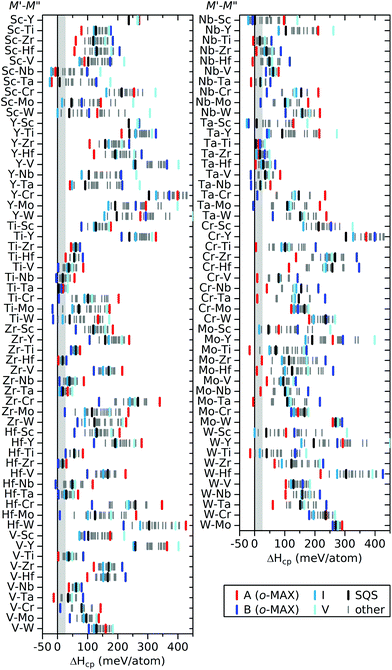 | ||
Fig. 5 Calculated formation enthalpy ΔHcp of the 413 MAX phase structures with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 composition of M′ 3 composition of M′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M′′ M′′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C, for chemically ordered o-MAX (A and B), order of type I and V (shown in Fig. 1b), additional (other) ordered configurations, and chemically disordered (SQS) configurations of M′ and M′′. C, for chemically ordered o-MAX (A and B), order of type I and V (shown in Fig. 1b), additional (other) ordered configurations, and chemically disordered (SQS) configurations of M′ and M′′. | ||
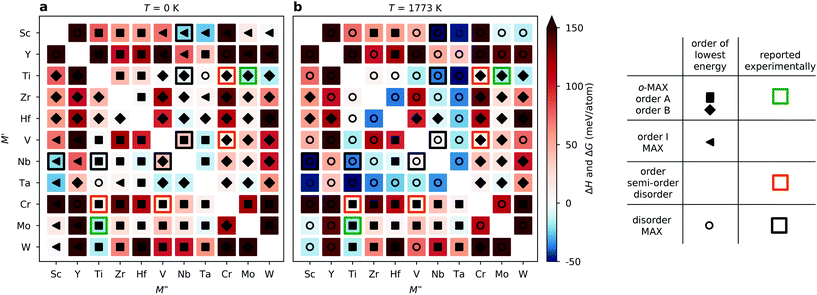
Again, we use a heatmap representation to visualize the thermodynamic stability and to determine whether chemical order or disorder is preferred at 0 K and at typical synthesis conditions (1773 K). Fig. 6a depicts ΔHcp at 0 K showing that a majority, 41 of 55, of the M′ and M′′ combinations are ordered in the o-MAX structure Reported o-MAX phases to date are identified as stable, Mo2Ti2AlC3,15 or close to stable for Cr2Ti2AlC3 and Cr2V2AlC3.17,46 11 combinations show preference for order of type I and only 3 show preference for disorder. Again, the reported disordered MAX phases are not captured by the 0 K results, and we therefore consider the contribution from the configurational entropy to the Gibbs free energy, using eqn (2) and (3), at a typical bulk synthesis temperature of 1773 K. Fig. 6b depicts the calculated ΔHcp (for o-MAX phases) or ΔGcp at 1773 K (for disordered MAX phases), depending on which ordering is of lowest energy. Now 32 out of 55 M′ and M′′ combinations are identified as disordered, and out of these 13 are predicted stable, including the MAX phases reported to date with disorder; (Ti0.5Nb0.5)4AlC3,47 (V0.5Cr0.5)4AlC3,48 and (Nb0.5V0.5)4AlC3.43 (Nb0.5Sc0.5)4AlC3 is one of the concluded stable disordered phases, and a chemically related phase, (Nb0.67Sc0.33)4AlC3, has recently been synthesized, albeit with a diverging stoichiometry compared to herein.44 23 phases are identified as o-MAX, with 4 being predicted stable, including the experimentally reported Mo2Ti2AlC3.15
Beyond the reported o-MAX we here predict three phases to be stable with preference for o-MAX ordering; Nb2Hf2AlC3, Mo2Ta2AlC3, and W2Ti2AlC3. An additional four systems are close to stable with preference for o-MAX ordering; Cr2Ta2AlC3, Mo2Zr2AlC3, Mo2Hf2AlC3 and Mo2Nb2AlC3. We also identify 12 systems which are stable and with preference for disorder at T = 1773 K, with a majority combining M′ and M′′ from groups 4 and 5.
Table 1 summarizes selected key results of the thermodynamic stability evaluation, where stable and close to stable 312 and 413 MAX phases with their preference for order or disorder are given. Comparing the experimentally reported phases and their calculated stability, the results are consistent. In addition, we also list hypothetical phases that are stable or close to stable, and provide information on whether or not chemical order or disorder of M′ and M′′ is to be expected.
| Phase | Stability criteria | Experimentally reported and here predicted | Predicted |
|---|---|---|---|
| a Reported with intermixing of V on Cr-site (Cr0.75V0.25)2VAlC2. b Reported with slight off-stoichiometric Cr2.5Ti1.5AlC3. c Reported with some intermixing between M sublattices (Cr0.7V0.3)2(V0.8Cr0.2)2AlC3. d Have also been reported as Ti2ZrAlC2 o-MAX.45,49 e Reported stoichiometry (Nb0.67Sc0.33)4AlC3. | |||
| o-MAX stable | ΔHcp < 0 | Cr2TiAlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
Sc2M′′AlC2, (M′′ = Nb, Ta, W) |
| T disorder > 1773 K | Mo2ScAl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
W2TiAlC2 | |
Mo2TiAlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
Nb2Hf2AlC3 | ||
Mo2Ti2AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Mo2Ta2AlC3 | ||
| W2Ti2AlC3 | |||
| o-MAX close to stable | 0 ≤ ΔHcp < +50 | Cr2VAlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Mo2M′′AlC2 (M′′ = Zr, Hf, V, Nb, Ta) |
| T disorder > 1773 K | Cr2Ti2AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
W2ScAlC2 | |
Cr2V2AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Cr2Ta2AlC3 | ||
| Mo2M′′2AlC3 (M′′ = Zr, Hf, Nb) | |||
| Disordered MAX | ΔGcp[1773 K] ≤ 0 | (Zr0.67Ti0.33)3AlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
(Ti0.67M′′0.33)3AlC2 (M′′ = Hf, V, Nb, Ta, Mo, W) |
| T disorder < 1773 K | (Ti0.67Zr0.33)3AlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
(Zr0.67M′′0.33)3AlC2 (M′′ = Hf, Nb, Ta) | |
(V0.67Ti0.33)3AlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
(Hf0.67M′′0.33)3AlC2 (M′′ = Ti, Zr, Nb, Ta) | ||
(Nb0.67Sc0.33)3AlC2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
(V0.67M′′0.33)3AlC2 (M′′ = Ta, Cr) | ||
(Ti0.5Nb0.5)4AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
(Nb0.67M′′0.33)3AlC2 (M′′ = Ti, Zr, Hf, Ta) | ||
(V0.5Cr0.5)4AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
(Ta0.67M′′0.33)3AlC2 (M′′ = Sc, Ti, Zr, Hf, V, Nb) | ||
(Nb0.5V0.5)4AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
(Sc0.5M′′0.5)4AlC3 (M′′ = Ta, Mo, W) | ||
(Nb0.5Sc0.5)4AlC3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
(Ti0.5M′′0.5)4AlC3 (M′′ = V, Ta) | ||
| (Zr0.5M′′0.5)4AlC3 (M′′ = Hf, Nb, Ta) | |||
| (Hf0.5M′′0.5)4AlC3 (M′′ = Nb, Ta) | |||
| (Ta0.5M′′0.5)4AlC3 (M′′ = V, Nb) | |||
The origin of out-of-plane chemical order and disorder in MAX phases
Previous studies of o-MAX phases claim that metals like Mo and Cr tend to avoid the unfavourable fcc stacking with C, whereas elements like Ti prefer such arrangement.15,24,25 It has also been shown that for the hypothetical Mo3AlC2, the traditional MAX phase structure with fcc arrangement of Mo and C is not lowest in energy (ΔHcp = +141 meV per atom). Instead, the Mo3C2-layer prefers an ABAB stacking closely related to WC (ΔHcp = +81 meV per atom).24 In some o-MAX phases, e.g., Mo2TiAlC2, the unfavourable fcc arrangement of Mo and C is broken by replacing Mo with Ti in the mid-layer (M′′ at site 2a), resulting in ΔHcp = −17 meV per atom. However, this is not valid for all o-MAX phases, e.g., Mo2ScAlC2.16 An additional explanation is therefore that M′ at site 4f, next to the Al layer, should be more electronegative than Al, resulting in fewer electrons available for populating antibonding Al–Al orbitals, which is energetically expensive.24To increase the understanding of the formation of ordered and disordered MAX phase configurations, and to test the previously suggested hypothesis for the origin of o-MAX, we choose to investigate trends in type of metal M′ next to the Al layer (site 4f for 312 and 413), type of metal M′′ sandwiched between C layers (site 2a for 312 and 4e for 413), and the metallic radius (r) and electronegativity (ρ) of M′, M′′ and A. For this analysis, only chemical order of type A (o-MAX) and chemical disorder is considered.
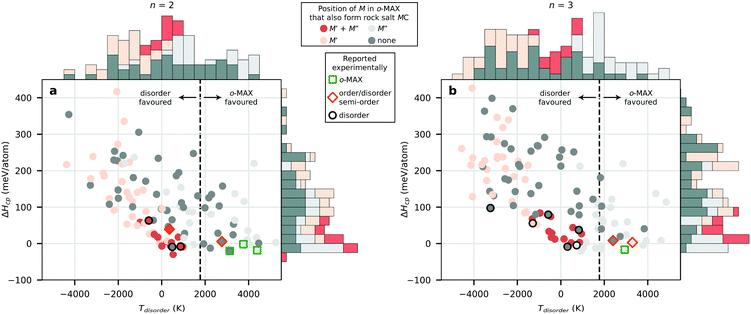
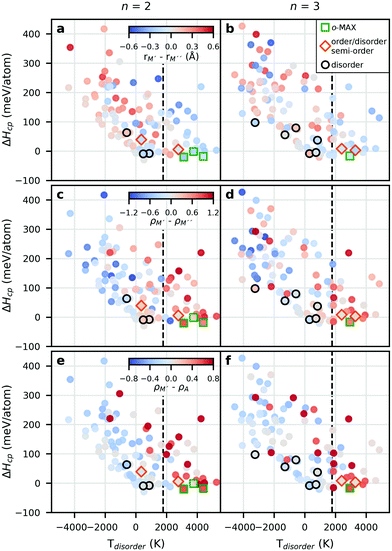
We start by looking at whether the metals M′ and M′′ in o-MAX also prefer formation of the binary MC in its rock salt structure. To be consistent, we herein use ΔHcp in the analysis instead of the isostructural formation enthalpy, ΔHiso, since the latter is biased from whether M prefer an fcc arrangement with C or not. This is most pronounced for the 413 system as seen in Fig. S2.† In Fig. 7, the calculated ΔHcp for o-MAX is shown as a function of the disorder temperature Tdisorder, where the colouring represents if M′ and/or M′′ in the o-MAX structure also form the binary rock salt MC. Note that for Tdisorder < 0, the disorderd MAX is lower in energy than o-MAX, whereas for Tdisorder > 0, the o-MAX is lower in energy. In addition, experimentally reported phases have been marked according to their reported order to aid in the interpretation. For both 312 and 413 o-MAX phases qualitatively similar results are found;
• When both M′ and M′′ in o-MAX also form MC (red), this results in Tdisorder close to zero, i.e., no or small energy difference between order and disorder, indicating preference for disorder at typical synthesis temperatures.
• When only M′ in o-MAX also form MC (light red), this results in Tdisorder < 0, i.e., disorder is lower in energy than order, clearly indicating preference for disorder.
• When only M′′ in o-MAX also form MC (light grey), this generally results in Tdisorder > 0, i.e., order is lower in energy than disorder at 0 K. When Tdisorder > 1773 K, preference for o-MAX is expected.
• When none of M′ and M′′ in o-MAX form MC (dark grey) we find −5000 K < Tdisorder < +5000 K. Hence, no general rule can be identified for this case.
The presented results partially support the previous statement that having a M′′ that also form a rock-salt MC is beneficial for o-MAX formation (Cr2TiAlC2 and Mo2Ti2AlC3), but this do not explain the formation of, e.g., Mo2ScAlC2.
Trends in ΔHcp for o-MAX as a function of the disorder temperature Tdisorder is shown in Fig. 8 for M′2M′′AlC2 (left) and M′2M′′2AlC3 (right). The colouring in the top panels represent the difference in size between M′ and M′′, where red represents rM′ > rM′′ and blue rM′ < rM′′. The mid panels display the difference in electronegativity between M′ and M′′, where red represents ρM′ > ρM′′ and blue ρM′ < ρM′′, while the bottom panels display the difference in electronegativity between M′ and Al, where red represents ρM′ > ρAl and blue ρM′ < ρAl. The values used for r and ρ are found in Table S5.†
Focusing on the region which indicates a preference for chemical order, i.e., Tdisorder > 1773 K, suggests that for a majority of phases M′ should be more electronegative than M′′ and with a size difference of M′ and M′′ being at most 0.2 Å. We also find that M′ should be more electronegative than Al. The latter is in line with previous work suggesting that having a M′ with larger electronegativity than Al results in fewer electrons available for populating antibonding Al–Al orbitals.24
Within the region where disorder is preferred, i.e., Tdisorder < 1773 K, and with 0 < ΔHcp < +50 meV per atom, we find hypothetical as well as experimentally reported disordered phases, with a difference in electronegativity of M′ and M′′ within 0.4, while the size difference of M′ and M′′ does not exceed 0.2 Å. The results also indicate that the difference in electronegativity of M′ and Al should be small.
The Hume-Rothery rules states that if there is a large enough difference in size and electronegativity between two atomic species, chemical order will be preferred, whereas for no or small differences disorder is favoured.50 Our results indicate that an explanation for order/disorder formation upon on alloying between M′ and M′′ within the 312 and 413 MAX phase structure is only consistent with the part of the Hume-Rothery rules related to electronegativity. When it comes to the size criteria for ordering, we find that a large size difference of M′ and M′′ within order of type A (o-MAX) is detrimental, with Tdisorder < 0 in general and ΔHcp ≫ 0. This is most pronounced for Y-based phases due to its large metallic radius (1.80 Å), which results in compressive stress in the Y-layers while the layers with smaller atoms will be under tensile stress. This is opposite to the in-plane ordered i-MAX phases,18,19,21–23 related to the 211 MAX phases, where a significant size difference between M′ and M′′ is required for the ordered formation of M′ and M′′ in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio within each M-layer.
1 ratio within each M-layer.
Based on herein presented result we propose guidelines for which M combination to use when targeting either chemical order (out-of-plane) or disorder in 312 and 413 MAX phases. Stable phases with preference for chemical order at typical bulk synthesis temperatures have mainly been identified for systems where the central M′′, sandwiched by C, also form rock-salt MC and with M′ = Cr and Mo next to the Al-layer. This is most pronounced for the 413 system but is also valid for the 312 systems. For the 312 systems we have also identified ordered phases with M′ = Sc next to the Al-layer. o-MAX is mainly preferred when M′ from group 6 (Cr, Mo, W) is combined with M′′ from groups 4 and 5 (Ti, Zr, Hf, V, Nb, Ta). Disorder of M′and M′′ is preferred when alloying metals from groups 3 to 5 (Sc, Ti, Zr, Hf, V, Nb, Ta).
A comparison of herein presented results with experimentally reported phases with order and/or disorder illustrates that ordered o-MAX phases can all be found within the region where we suggest that order is preferred, i.e., Tdisorder > 1773 K, while disordered MAX is found for Tdisorder < 1773 K. Some M′ and M′′ combinations have been reported both as ordered and disordered, e.g., Cr2V2AlC3 and (Cr0.5V0.5)4AlC3 (choice of notation indicating order/disorder), which have been reported as o-MAX with some degree of intermixing between the two M-sites and as disordered.17,48 We have previously shown that o-MAX phases can be stable both upon limited intermixing of M′ and M′′, and at off-stoichiometries.15,24 Ti2ZrAlC2 or (Ti0.67Zr0.33)3AlC2 is another example where both order45,49 and disorder42,51 have been claimed. Our results indicate that Ti and Zr prefer disorder or possibly semi-order, based on the moderate Tdisorder = 357 K combined with a similar electronegativity of Ti and Al. It should be noted that within this study, only out-of-plane order have been considered, where each M-layer only consists of one elemental type only. Off-stoichiometries for o-MAX have been demonstrated both experimentally and theoretically, and shows that deviation from the ideal o-MAX composition is possible while still retaining the overall chemical order.15,17,24 Impact from off-stoichiometries, intermixing between M′ and M′′ layers, and vacancies at M, Al or C sites have previously been demonstrated for a few M′ and M′′ combinations to have minor influence on the qualitative results.24 The possibility of in-plane order of M′ and M′′, similar to i-MAX phases,18,19 in higher order MAX phases, 312 and 413, is beyond the scope of this work and a topic for future studies.
There are already MXenes realized from o-MAX phases16,25 and the range of materials predicted in the present work suggest that the number of chemically ordered as well as disordered MXenes, from o-MAX or solid solution 312 and 413 MAX phases, respectively, can be increased. This allows tuning of the MXene chemistry through one or two metals in the outmost surface layers, and alteration of fundamental properties through choice of metal embedded within in the MXene.
Conclusion
In conclusion, the present study demonstrates that formation of chemically ordered o-MAX phases is mainly governed by having a M′ (the metal layer closest to the A-layer) that do not form rock-salt MC and with an electronegativity larger than Al. Preference for chemical disorder is achieved when the difference in size and electronegativity of M′ and M′′ is small combined with minor differences in electronegativity of M′ and Al. Through a systematic theoretical study of phase stability of quaternary MAX phases upon alloying between M′ and M′′ from groups 3 to 6 (Sc, Y, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, and W) in 312 (M′2M′′AlC2 composition) and 413 (M′2M′′2AlC3 composition) structures, we confirm all synthesized phases to date with chemical order or disorder, and identify 7 stable (10 close to stable) hypothetical o-MAX phases, and 38 MAX phases with preference for disorder. These phases remain to be verified, and synthesis experiments are encouraged. We also propose guidelines for which M′ and M′′ combinations to use in search for chemically ordered o-MAX; M′ from group 6 (Cr, Mo, W) together with M′′ from groups 3–5 (Sc, Ti, Zr, Hf, V, Nb, Ta). Correspondingly, disordered MAX phases are obtained by combing M′ and M′′ within groups 3–5 (Sc, Ti, Zr, Hf, V, Nb, Ta). This study shows that adding a fourth element to form ordered and disordered quaternary MAX phases allows novel elemental combinations, in materials not yet synthesized, for potentially tuneable and advantageous properties. Moreover, based on o-MAX phases as parent materials for corresponding MXenes, we also expect that the range of attainable MXene compositions will be expanded.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
J. R. acknowledge support from the Knut and Alice Wallenberg (KAW) Foundation for a Fellowship Grant and Project funding (KAW 2015.0043), and from the Swedish Foundation for Strategic Research (SSF) for Project Funding (EM16-0004). The Swedish Research Council is also gratefully acknowledged through Project 642-2013-8020. The calculations were carried out using supercomputer resources provided by the Swedish National Infrastructure for Computing (SNIC) at the National Supercomputer Centre (NSC), the High Performance Computing Center North (HPC2N), and the PDC Center for High Performance Computing.Notes and references
- W. Jeitschko, H. Nowotny and F. Benesovsky, Monatsh. Chem., 1963, 94, 672–676 CrossRef CAS.
- W. Jeitschko, H. Nowotny and F. Benesovsky, Monatsh. Chem., 1964, 95, 178–179 CrossRef CAS.
- M. W. Barsoum and T. El-Raghy, J. Am. Ceram. Soc., 1996, 79, 1953–1956 CrossRef CAS.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201–281 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. Man Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS PubMed.
- F. L. Meng, Y. C. Zhou and J. Y. Wang, Scr. Mater., 2005, 53, 1369–1372 CrossRef CAS.
- T. Cabioch, P. Eklund, V. Mauchamp, M. Jaouen and M. W. Barsoum, J. Eur. Ceram. Soc., 2013, 33, 897–904 CrossRef CAS.
- A. S. Ingason, A. Mockute, M. Dahlqvist, F. Magnus, S. Olafsson, U. B. Arnalds, B. Alling, I. A. Abrikosov, B. Hjörvarsson, P. O. Å. Persson and J. Rosen, Phys. Rev. Lett., 2013, 110, 195502 CrossRef CAS PubMed.
- V. Saltas, D. Horlait, E. N. Sgourou, F. Vallianatos and A. Chroneos, Appl. Phys. Rev., 2017, 4, 041301 Search PubMed.
- Z. Liu, E. Wu, J. Wang, Y. Qian, H. Xiang, X. Li, Q. Jin, G. Sun, X. Chen, J. Wang and M. Li, Acta Mater., 2014, 73, 186–193 CrossRef CAS.
- B. Anasori, J. Halim, J. Lu, C. A. Voigt, L. Hultman and M. W. Barsoum, Scr. Mater., 2015, 101, 5–7 CrossRef CAS.
- B. Anasori, M. Dahlqvist, J. Halim, E. J. Moon, J. Lu, B. C. Hosler, E. N. Caspi, S. J. May, L. Hultman, P. Eklund, J. Rosén and M. W. Barsoum, J. Appl. Phys., 2015, 118, 094304 CrossRef.
- R. Meshkian, Q. Tao, M. Dahlqvist, J. Lu, L. Hultman and J. Rosen, Acta Mater., 2017, 125, 476–480 CrossRef CAS.
- E. N. Caspi, P. Chartier, F. Porcher, F. Damay and T. Cabioch, Mater. Res. Lett., 2015, 3, 100–106 CrossRef.
- Q. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. Å. Persson and J. Rosen, Nat. Commun., 2017, 8, 14949 CrossRef PubMed.
- M. Dahlqvist, J. Lu, R. Meshkian, Q. Tao, L. Hultman and J. Rosen, Sci. Adv., 2017, 3, e1700642 CrossRef PubMed.
- L. Chen, M. Dahlqvist, T. Lapauw, B. Tunca, F. Wang, J. Lu, R. Meshkian, K. Lambrinou, B. Blanpain, J. Vleugels and J. Rosen, Inorg. Chem., 2018, 57, 6237–6244 CrossRef CAS PubMed.
- M. Dahlqvist, A. Petruhins, J. Lu, L. Hultman and J. Rosen, ACS Nano, 2018, 12, 7761–7770 CrossRef CAS PubMed.
- R. Meshkian, M. Dahlqvist, J. Lu, B. Wickman, J. Halim, J. Thörnberg, Q. Tao, S. Li, S. Intikhab, J. Snyder, M. W. Barsoum, M. Yildizhan, J. Palisaitis, L. Hultman, P. O. Å. Persson and J. Rosen, Adv. Mater., 2018, 30, 1706409 CrossRef PubMed.
- J. Lu, A. Thore, R. Meshkian, Q. Tao, L. Hultman and J. Rosen, Cryst. Growth Des., 2017, 17, 5704–5711 CrossRef CAS.
- M. Dahlqvist and J. Rosen, Phys. Chem. Chem. Phys., 2015, 17, 31810–31821 RSC.
- B. Anasori, Y. Xie, M. Beidaghi, J. Lu, B. C. Hosler, L. Hultman, P. R. C. Kent, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2015, 9, 9507–9516 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- Y. Ze-Jin, L. Rong-Feng, G. Qing-He, X. Heng-Na, X. Zhi-Jun, T. Ling, J. Guo-Zhu and G. Yun-Dong, Sci. Rep., 2016, 6, 34092 CrossRef PubMed.
- M. Dahlqvist, R. Meshkian and J. Rosen, Data Brief, 2017, 10, 576–582 CrossRef PubMed.
- A. Zunger, S. H. Wei, L. G. Ferreira and J. E. Bernard, Phys. Rev. Lett., 1990, 65, 353–356 CrossRef CAS PubMed.
- M. Dahlqvist, B. Alling, I. A. Abrikosov and J. Rosén, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 024111 CrossRef.
- M. Dahlqvist, B. Alling and J. Rosén, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 220102 CrossRef.
- P. Eklund, M. Dahlqvist, O. Tengstrand, L. Hultman, J. Lu, N. Nedfors, U. Jansson and J. Rosén, Phys. Rev. Lett., 2012, 109, 035502 CrossRef PubMed.
- A. S. Ingason, A. Petruhins, M. Dahlqvist, F. Magnus, A. Mockute, B. Alling, L. Hultman, I. A. Abrikosov, P. O. Å. Persson and J. Rosen, Mater. Res. Lett., 2014, 2, 89–93 CrossRef CAS.
- A. Mockute, M. Dahlqvist, J. Emmerlich, L. Hultman, J. M. Schneider, P. O. Å. Persson and J. Rosen, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 094113 CrossRef.
- A. Mockute, P. O. Å. Persson, F. Magnus, A. S. Ingason, S. Olafsson, L. Hultman and J. Rosen, Phys. Status Solidi RRL, 2014, 8, 420–423 CrossRef CAS.
- E. Zapata-Solvas, M. A. Hadi, D. Horlait, D. C. Parfitt, A. Thibaud, A. Chroneos and W. E. Lee, J. Am. Ceram. Soc., 2017, 100, 3393–3401 CrossRef CAS.
- M. Naguib, G. W. Bentzel, J. Shah, J. Halim, E. N. Caspi, J. Lu, L. Hultman and M. W. Barsoum, Mater. Res. Lett., 2014, 2, 233–240 CrossRef.
- J. Halim, J. Palisaitis, J. Lu, J. Thörnberg, E. J. Moon, M. Precner, P. Eklund, P. O. Å. Persson, M. W. Barsoum and J. Rosen, ACS Appl. Nano Mater., 2018, 1, 2455–2460 CrossRef CAS.
- B. Tunca, T. Lapauw, O. M. Karakulina, M. Batuk, T. Cabioc'h, J. Hadermann, R. Delville, K. Lambrinou and J. Vleugels, Inorg. Chem., 2017, 56, 3489–3498 CrossRef CAS PubMed.
- Z. Liu, L. Zheng, L. Sun, Y. Qian, J. Wang and M. Li, J. Am. Ceram. Soc., 2014, 97, 67–69 CrossRef CAS.
- L. Zheng, J. Wang, X. Lu, F. Li, J. Wang and Y. Zhou, J. Am. Ceram. Soc., 2010, 93, 3068–3071 CrossRef CAS.
- Y. Zhou, F. Meng and J. Zhang, J. Am. Ceram. Soc., 2008, 91, 1357–1360 CrossRef CAS.
- R. Arróyave, A. Talapatra, T. Duong, W. Son and M. Radovic, Mater. Res. Lett., 2018, 6, 1–12 CrossRef.
- W. Hume-Rothery, R. E. Smallman and C. W. Haworth, The structure of metals and alloys, The Institute of Metals, London, 1969 Search PubMed.
- S. V. Konovalikhin, S. A. Guda and D. Y. Kovalev, Inorg. Mater., 2018, 54, 953–956 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr08675g |
| This journal is © The Royal Society of Chemistry 2020 |