Multifunctional inorganic nanomaterials for energy applications†
Huilin
Wang
 ab,
Xitong
Liang
ab,
Xitong
Liang
 ab,
Jiutian
Wang
ab,
Jiutian
Wang
 ab,
Shengjian
Jiao
ab,
Shengjian
Jiao
 ab and
Dongfeng
Xue
ab and
Dongfeng
Xue
 *ab
*ab
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China. E-mail: dongfeng@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei 230026, China
First published on 18th November 2019
Abstract
Our society has been facing more and more serious challenges towards achieving highly efficient utilization of energy. In the field of energy applications, multifunctional nanomaterials have been attracting increasing attention. Various energy applications, such as energy generation, conversion, storage, saving and transmission, are strongly dependent upon the electrical, thermal, mechanical, optical and catalytic functions of materials. In the nanoscale range, thermoelectric, piezoelectric, triboelectric, photovoltaic, catalytic and electrochromic materials have made major contributions to various energy applications. Inorganic nanomaterials’ unique properties, such as excellent electrical and thermal conductivity, large surface area and chemical stability, make them highly competitive in energy applications. In this review, the latest research and development of multifunctional inorganic nanomaterials in energy applications were summarized from the perspective of different energy applications. Furthermore, we also illustrated the unique functions of inorganic nanomaterials to improve their performances and the combination of the functions of nanomaterials into a device. However, challenges may be traced back to the limitations set by scaling the relations between multifunctional inorganic nanomaterials and energy devices.
1. Introduction
Water, food and energy are always needed by society.1,2 In fact, the history of human civilization is also a history of storing and utilizing energy.3 Today, the world's population is about 7 billion and is estimated to grow to 9 billion by 2050 and then to about 10 billion by 2100.4 Such rapid population and also economic growth will place additional demands on the global energy supply. The International Energy Agency has projected that the world's energy demand will increase from about 12 billion tonne oil equivalents (t.o.e.) in 2009 to either 18 billion t.o.e. or 17 billion t.o.e. by 2035 under their current policy or new policy scenarios, respectively.5 Carbon-dioxide emissions are expected to increase from 29 gigatonnes per year to 43 Gt per year or 36 Gt per year under the current and new policies, respectively. The data given above also reflect the fact that energy shortage and environmental deterioration resulting from insufficient fossil fuel supplies and increasing consumption have become two major global problems for human beings.6 As a result, developing new technologies to make full use of abundant “green” energy sources, such as solar energy, mechanical energy and thermal energy, is an effective way to meet our long-term energy needs and achieve sustainable environmental development.7 The use of energy in the twenty-first century must also be sustainable. Access to clean, affordable and reliable energy has been a cornerstone of the world's increasing prosperity and economic growth.It is widely acknowledged that human life is closely related to various energy applications, which are needed to address the global challenge of producing plentiful and sustainable energy for the future. The various energy applications include energy generation, conversion, storage, saving and transmission applications.8 This review covers a wide range of applications in the renewable and conventional energy sectors. Looking for new ways of generating electricity in addition to traditional fossil fuel combustion remains a vibrant topic for addressing the demands on energy supply, in which the harvester generates electrical energy form heat, light and mechanical vibrations. Electrical energy is subsequently rectified, conditioned and stored within capacitors or batteries for various applications, such as wireless and self-powered sensors or low-power electronics.9 In addition to energy generation, human society also relies on energy conversion, which is the transformation of energy from forms provided by nature to forms that can be used by humans. In recent years, considerable attention has been devoted to certain direct energy conversion devices, notably solar cells and fuel cells.10 Although there are several types of renewable energies, such as wind, solar, tidal, biomass and geothermal energies, they are inherently intermittent and generate huge fluctuations when they are incorporated into the power grid. Therefore, we need advanced energy storage devices to make full use of the various energy sources.7 Before shifting from a fossil fuel economy to one based on renewable technologies, there is clearly a pressing need to significantly improve our ability to store energy.7 Recently, a lot of research has gone into batteries and supercapacitors. Furthermore, an additional major challenge is that the solar, wind and hydro energy sources are often located far from densely populated areas that are the customers for the renewable energy utilization, which means that electricity generation from these distant renewable energy sources must be combined with cost-effective energy transmission systems.11
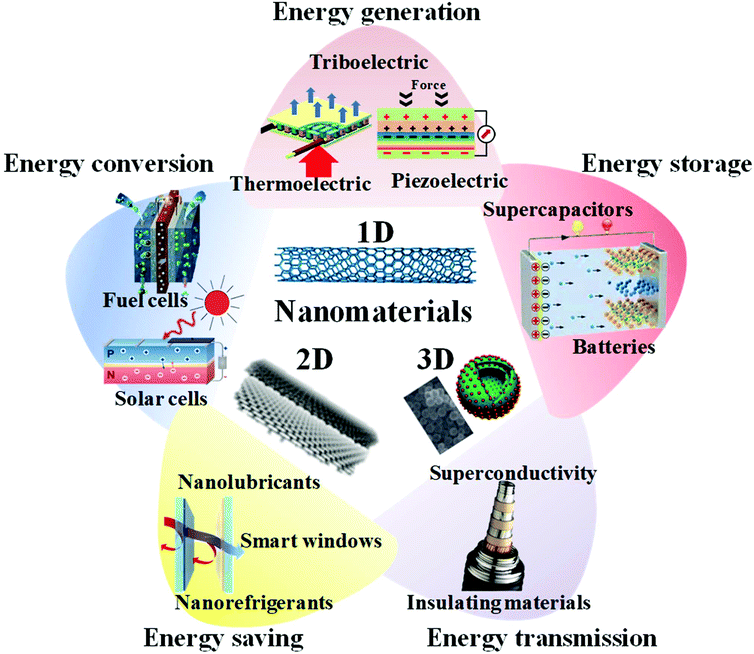
Multifunctional materials are a type of materials or material-based system that are designed to perform multiple tasks via a judicious combination of various functions. They are expected to deliver system-level efficiency beyond their individual parts. Generally, their function may participate concurrently or sequentially, and can be implemented on the same length-scale or hierarchically organized. Often, applications demand the multifunctional features of materials, as shown in Scheme 1. These remarkable features may include special optical, magnetic and electrical properties, among many others, which could potentially have great impacts on electronics, spintronics, medical applications and other devices.12 Integrating different functions in one material system is a fundamental challenge. Such a system would need to be carefully designed to perform multiple responsibilities through combinations of different functions. Typically, each function contributes a distinct physical or chemical process that can deliver system-level improvements beyond the individual parts.13 Batteries require electrode materials with high electrochemical reactivity, good electrochemical reaction reversibility, excellent chemical stability and low toxicity. The additional functionality of the battery needs materials with special features. For example, in flexible batteries, the electrode must have a flexible function that maintains high performance even under severe bending. In solid-state batteries, the electrolyte material needs to have high ionic conductivity to ensure the ion transport rate inside the battery, which is not available in general solid material. In the nanoscale range, thermoelectric, piezoelectric, triboelectric, photovoltaic and catalytic materials have made major contributions to various energy applications. Due to the unique properties of inorganic nanomaterials, such as excellent electrical and thermal conductivity, large surface area and chemical stability,14 multifunctional inorganic nanomaterials play a key role in developing advanced energy applications with enhanced performance.14,15 In energy generation applications, nanoscale materials have helped to nearly double the performance of thermoelectric materials.16 In energy conversion applications, nanostructuring is an advanced strategy for increasing the active sites of catalytic materials.17 These recent advances have the potential to propel further the prospects of tuning the hybrid properties at the nanoscale, which could lead to next generation materials for energy applications. Multifunctional nanomaterials research is the study of how the structures of materials determine their properties, including their design and fabrication.
The use of multifunctional inorganic nanomaterials for energy applications faces significant challenges. These challenges are mainly from the exploration of novel materials, such as exploring new material structure systems, excavating new properties of existing structures and improving the performance of existing materials. Nanomaterials present chemical, physical and electrical properties that change as a function of the size and shape of the material (Fig. 1).18,19 Nanomaterials have very high proportions of surface atoms and they are often subject to property variation as a function of size, owing to quantum confinement effects.18 Taking metals as an example, the evolution of energy levels is presented in Fig. 1b from that of individual and separated atoms to that in clusters of 2, 5, 20 and 1020 atoms.19 The electronic state energy and electron delocalisation degree are shown in Fig. 1c. Valence electrons are able to move between the occupied and unoccupied orbits easily within the valence band, offering different levels of semiconductivity.19 In general, the study of multifunctional inorganic nanomaterials at the nanoscale provides richer possibilities, broader perspective, and more flexible approaches to overcome these challenges.
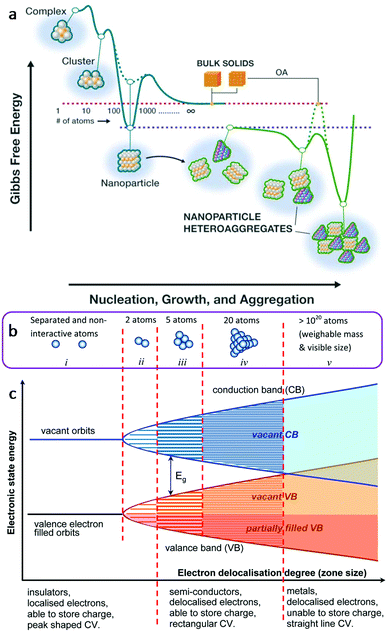 | ||
| Fig. 1 (a) Conceptual free-energy landscape for the formation and interactions that define the typical state of nanomaterials in the environment. This figure was reproduced from ref. 18 with permission from The American Association for the Advancement of Science, copyright 2019. (b) Schematic illustrations of the band model for chemical bonding between metal atoms. (c) Corresponding energy levels of the valence electrons as a function of the degree of delocalization of valence electrons in the cluster of metal atoms. This figure was reproduced from ref. 19 with permission from Taylor & Francis Group, copyright 2017. | ||
2. Energy applications
2.1 Energy generation applications
Energy generation, also known as energy harvesting, aims to convert ambient forms of energy, such as mechanical motion and heat, which are otherwise wasted, into useful energy (in many cases electrical energy).9 This can make a valuable contribution to solving the issues of the expected fossil fuel shortage in the near future as well as could help to reduce emissions and toxic waste into the environment.20 In this section, the energy-generation approaches cover thermoelectric, piezoelectric and triboelectric effects. The realization of these approaches depend on the multifunction of materials. Over the past decades, scientists have focused on the research of multifunctional materials, which has promoted the development of the energy-generation field. However, the performances of devices, such as the output voltage, conversion efficiency and stability, still cannot meet practical demands. The development of nanotechnology has given rise to the dawn of resolving these challenges. When the size of materials is reduced to the nanometre, many of their properties, such as electrical conductivity, thermal conductivity, dielectric constant and mechanical quality factor, change due to unique superficial effects, bulk effects, quantum dimension effects and macroscopic quantum tunnelling effects. Therefore, multifunctional nanomaterials are of great significance for energy generation.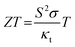 | (1) |
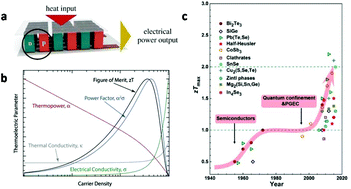 | ||
| Fig. 2 (a) Schematic of a TE module made from a thermocouple of p- and n-type thermoelectric legs. This figure was reproduced from ref. 21 with permission from John Wiley and Sons, copyright 2019. (b) Schematic showing the interdependence of the various TE properties with the carrier density (over three orders of magnitude), illustrating the challenge of trying to optimize the material ZT. The shapes of the individual curves are extracted from actual data on single-walled carbon nanotube networks. This figure was reproduced from ref. 16 with permission from John Wiley and Sons, copyright 2018. (c) Evolution of the maximum ZT values for some typical TE materials. This figure was reproduced from ref. 22 with permission from John Wiley and Sons, copyright 2017. | ||
It is noteworthy that these property parameters are dependent on each other. Since typical TE devices are based on the electromotive extraction and diffusion of the majority carriers, either electrons or holes, optimization of the parameters is related to the carrier concentration. As shown in Fig. 2b, with the increase in carrier density, the electrical conductivity increases, which is beneficial for ZT materials. However, the Seebeck coefficient decreases and the thermal conductivity increases, which are detrimental to ZT. At a certain temperature, a maximum ZT value of the material may be reached by tuning the interdependence of σ, S and κt.16
Classical theories and experiments have shown that nanomaterials can offer dramatic improvements to the ZT. The earliest theoretical study on thermoelectric nanomaterials was carried out by Hicks and Dresselhaus in 1993. They suggested that both nanoscale interfaces and quantum-confined nanomaterials would greatly improve the ZT.23,24 Subsequent experimental results confirmed that nanomaterials have a nearly doubled ZT value to bulk materials. Among numerous thermoelectric nanomaterials, chalcogenides, such as tin selenide, lead telluride and bismuth telluride, and carbon-based compounds, such as carbon nanotubes and fullerene, have shown advantages and attracted extensive interests (Fig. 2c).
Tin selenide (SnSe) has sparked much attention since the discovery of its record ZT of 2.6 ± 0.3 at 923 K along the b-axis of a single crystal. However, due to the special demands of crystal growth techniques, the prospective high cost for production, and undesirable mechanical properties, SnSe single crystals are not well-suited for thermoelectric devices. Therefore, polycrystalline and doped SnSe has become a promising alternative candidate for single crystals. A thin film of SnSe nanosheets was fabricated by using a simple thermal evaporation method, and exhibited a ZT of 0.055 at 501 K.25 Despite the value being lower than for the SnSe single crystal, it was the highest reported ZT for a SnSe film above 70 K. Such an excellent thermoelectric performance was due to the unprecedentedly low κt (0.08 W m−1 K−1 in the temperature range between 375 and 450 K), which was primarily due to the reduction of the lattice component.
A p-type polycrystalline Cd-doped SnSe was synthesized by combining cation vacancies and through localized-lattice engineering, and its ZT was approximately as high as 1.7 at 823 K. A high cation vacancy concentration of about 2.9% contributed to a high hole carrier concentration of about 2.6 × 1019 cm−3, which in turn led to a high S2σ of about 6.9 μW cm−1 K−2. Meanwhile, the doped Cd resulted in massive nanoscale crystal imperfections, including dislocations, intensive local lattice distortions and point defects, which contributed to a low κt of approximately 0.33 W m−1 K−1.26 Cation-doped SnSe nanomaterials could enhance the TE performance of p-type polycrystalline SnSe by Ag/Na dual-doping and Ag8SnSe6 (STSe) nanoprecipitates. The Ag/Na dual-doped p-type polycrystalline SnSe led to a two orders of magnitude increase in the carrier concentration, which in turn resulted in a sharp enhancement of σ and a high Seebeck coefficient. Moreover, an ultralow κt of below 0.3 W m−1 K−1 was realized for the Sn0.99Na0.01Se-STSe sample at 773 K, which represented an approximately 20% reduction compared to the pure SnSe. This was caused by the nanostructured SnSe matrix with dispersed nanoprecipitates of the compound Ag8SnSe6, which further strengthened the scattering of phonons. Consequently, Sn0.99Na0.01Se-STSe exhibited a high peak ZT of 1.33 at 773 K and an outstanding average ZT (ZTave) of 0.91 in the temperature range of 423–823 K.27
Lead telluride (PbTe) is a state-of-the-art thermoelectric material at mediate temperature. The highly symmetric crystal structure assures it a high band degeneracy and thus a decent effective mass. The bond inharmonicity caused by the slight displacement of Pb atoms determines that the lattice thermal conductivity can be maintained at a relatively low value. Tremendous efforts have been devoted to improving the thermoelectric performance of PbTe-based materials. A PbTe-4%InSb composite was fabricated through introducing InSb multi-nanophases to the n-type PbTe matrix.28 A highest ZT of 1.83 was obtained for the sample PbTe-4%InSb at 773 K. The highest average ZT reached about 1.0 for the sample PbTe-5%InSb, which is a new record for n-type PbTe-based materials. The outstanding performance resulted from the simultaneous enhancement in S2σ and reduction in κt across the entire temperature range. The enhanced S2σ was mainly due to the significantly increased absolute Seebeck coefficient via multiphase energy filtering effects, and the reduced κt can be understood via phonon scattering by the incorporated multi-nanophases.29
A Sb-doped and GeTe-alloyed n-type thermoelectric nanomaterial was reported. GeTe alloying enlarged the band gap and electron effective mass, leading to a considerable enhancement of the Seebeck coefficient to −280 μV K−1 at 673 K. Meanwhile the high density of point defects resulting from the supersaturated state led to greatly decreasing κt to 0.56 W m−1 K−1 at 573 K. Moreover, the formation of nanostructures could further improve the S2σ and reduce κt simultaneously. As a result, the Pb0.988Sb0.012Te-13%GeTe-nano achieved a ZT value of 1.38 at 623 K and a high average ZTavg value of about 1.04 in the temperature range of 300–773 K.30
In addition to cationic doping, the enhanced TE performance of anionic-doped PbTe nanomaterials has also been reported. As an example, a high ZT of approximately 1.4 at 900 K was realized in 1.25% Sb-contained and 12% S-introduced PbTe. The introduction of S opened the band gap of PbTe, which suppressed bipolar conduction and increased the electron concentration and electrical conductivity. Furthermore, point defects induced second phase nanostructuring, which led to a strong reduction of the κt to approximately 0.5 W m−1 K−1 at 900 K. An increase in the Seebeck coefficient of PbTe was also observed by S replacement for Te, due to the larger effective mass of electrons of PbS compared with that of PbTe. All these optimizations contributed to the improvement of the ZT.31
Following the success of nanotechnology in selenide and telluride thermoelectric materials, sulfide nanoparticles were also studied. An orthorhombic@cubic core–shell nonstoichiometric Cu5FeS4 icosahedral nanoparticle was reported, which contained high-density twin boundaries in the form of fivefold twins. Due to inclusion of the high-density twin boundaries and a tuned fraction of Fe-deficient cubic-structured Cu5FeS4, the thermal and electrical transport properties were synergistically optimized. The S2σ was enhanced to about 0.39 mW m−1 K−2 at 710 K. Meanwhile, the κt maintained a very low value of about 0.43 W m−1 K−1 within the whole temperature range from 300 K to 710 K. These outstanding properties of Cu5FeS4 icosahedral nanoparticles resulted in an enhanced ZT value of about 0.62 at 710 K.32
Carbon-based nanomaterials, including carbon nanotubes, graphene and fullerene, are other potential thermoelectric materials in virtue of their unique electronic structures and excellent mechanical properties. A highly pure semiconducting single-walled carbon nanotube (s-SWCNT) network with equally large n- and p-type thermoelectric performance metrics was successfully fabricated. The results pointed to strong correlations between the ultimate power factor and the electrical conductivity, both of which also correlated well with the diameter of the bundles within the s-SWCNT networks. The κt appeared to decrease with decreasing the s-SWCNT diameter, leading to a peak material ZT of approximately 0.12 with diameters in the range of 1.0 nm.33
The in situ growth of Cu2Se on the surface of CNTs has also been studied. A series of Cu2Se/CNT hybrid materials were fabricated. Due to the high degree of homogeneously dispersed molecular CNTs inside the Cu2Se matrix, large reductions in lattice thermal conductivity and carrier concentration were observed, leading to a record-high ZT of 2.4 at 1000 K.
TiS2/n-type fullerene hybrid films integrate mechanical flexibility and outstanding thermoelectric properties together. Assembling 0D C60 nanoparticles onto 2D TiS2 nanosheets not only significantly increased the Seebeck coefficient to about −130 mV K−1 and thereby the S2σ to about 375 mW m−1 K−2, but also reduced the κt of TiS2 to about 0.39 W m−1 K−1. The resultant TiS2/C60 hybrid films showed a ZT of approximately 0.3 at 400 K, far superior to the state-of-the-art flexible n-type thermoelectric materials.34
Other multifunctional inorganic nanomaterials have also shown fascinating potential in the TE field. With the development of nanotechnology, some multifunctional nanomaterials have significantly improved the performance of TE materials. With the help of high-throughput calculations, the materials selection process can be accelerated and new nanomaterials with highly efficient TE performance may be identified.22
The electric energy available from piezoelectric conversion can be calculated based on a compressive force (F). Let us consider a piezoelectric material with area (A) and thickness (t). The induced voltage (V) is defined as the ratio of surface polarization charge (Q) to the capacitance (C) of the material.
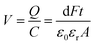 | (2) |
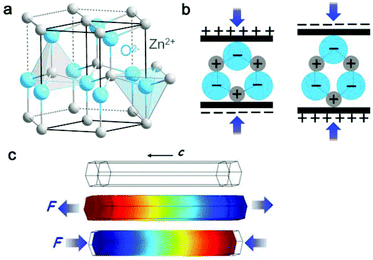 | ||
| Fig. 3 (a) Atomic model of the wurtzite-structured ZnO. (b) Piezoelectric properties and the different piezopotentials in the tension and compression modes of the material. (c) Numerical calculation of the piezoelectric potential distribution in a ZnO nanowire under axial strain. This figure was reproduced from ref. 36 with permission from John Wiley and Sons, copyright 2016. | ||
Typical piezoelectric materials include zinc oxide, lead zirconate titanate (PZT) and barium titanate (BaTiO3, BTO). The operation of a ZnO nanogenerator (NG) depends on a coupling of the piezoelectric and semiconducting properties. Since the first ZnO NG was demonstrated in 2006 by deforming a single ZnO nanowire with an atomic force microscopy tip, various great progresses, such as lateral ZnO nanowires, vertical ZnO nanowires and ZnO fibres, have been made to broaden the practical applications of ZnO NG.
A novel approach was developed by combining surface-coating and plasma-etching techniques to enhance the mechanical reliability of a Kevlar microfibre–ZnO nanowires hybrid structure for a piezoelectric nanogenerator (PENG).37 The enhanced microfibre–nanowire hybrid structure showed high flexibility, robustness and durability. Moreover, the enhanced ZnO nanowires-covered fibre was conducive to improving the stability of the PENG. The open-circuit voltage and short-circuit current of the PENG were 1.8 mV and 4.8 pA, respectively. Also, no decay appeared in the output performance for the enhanced PENG after 3600 s of operation.
In previous studies, most 2D transition-metal dichalcogenides (TMD) materials exhibit piezoelectric properties, unlike their bulk parent crystal. Remarkably, according to density-functional theory, the calculation of the piezoelectric coefficient for monolayer MoS2 revealed that the monolayer structure exhibited a stronger piezoelectric coupling than the bulk wurtzite-structured materials.
A sulfur-vacancy-passivated monolayer MoS2 PENG was proposed. The S vacancies were effectively passivated by using the S-treatment process on the pristine MoS2 surface. The S vacancy site had a tendency to covalently bond with S functional groups. Therefore, by capturing free electrons, a S atom forms a chemisorbed bond with the S vacancy site of MoS2. S treatment reduced the charge-carrier density of the monolayer MoS2 surface, thus the screening effect of piezoelectric polarization charges by free carriers was significantly revealed. As a result, the output peak current and voltage of the S-treated monolayer MoS2 nanosheet PENG were increased by more than 3 times (100 pA) and 2 times (22 mV), respectively.38
Piezoelectric ceramics have played an essential role in technology developments in many areas since the 1950s due to the extensive research and development of high-performance piezoelectric materials. PZT ceramics, excellent piezoelectric materials that have been widely used in piezoelectric electronic devices, have different electrical properties when their compositions are tailored with additives. However, increasing health and environmental concerns about the toxicity of lead in PZT ceramic materials have stimulated the search for high-performance lead-free piezoelectric materials. Potassium–sodium niobate (KNN) ceramics have been identified as one of the most promising lead-free candidates owing to their relatively good comprehensive performance. However, the deliquescence and alkali-oxide volatility deteriorated the piezoelectric properties of KNN-based ceramics. A novel (1 − x − y)K1−wNawNb1−zSbzO3−yBaZrO3−xBi0.5K0.5HfO3 ternary system exhibited a superior piezoelectric coefficient (d33 = 570 ± 10 pCN−1), the highest value reported to date in KNN-based ceramics. This high d33 value could be ascribed to the co-existence of nanoscale strain domains (1–2 nm) and a high density of ferroelectric domain boundaries.39
Oppositely charged surfaces with a changing gap distance can be viewed as a capacitor with varying capacitance. As shown in Fig. 4, this perspective gave a more intuitive representation of the TENG using the capacitor model, whose current is given by
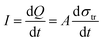 | (3) |
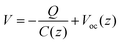 | (4) |
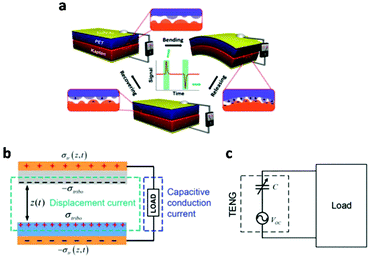 | ||
| Fig. 4 Theoretical models of a TENG. (a) Schematic illustration of the first TENG and its operation cycle. (b) The displacement current model of a contact-separation-mode TENG. (c) The equivalent electrical circuit model of a TENG. This figure was reproduced from ref. 42 with permission from John Wiley and Sons, copyright 2019. | ||
Along with Ohm's law, this capacitive model of a TENG is the theoretical tool that enables the study, design and optimization of TENG.
In principle, any material with distinct charge affinity can be used to construct a TENG, which results in a broad range of materials at opposite ends of the triboelectric series capable of high performance. Silver nanowires (AgNWs) were composited on the surface of PVDF as the triboelectric layers, achieving the development of high-performance TENGs. The addition of AgNWs to PVDF promoted the formation of the polar crystalline β-phase by introducing electrostatic interactions between the surface charges of the nanowires and the dipoles of the PVDF chains. Meanwhile the ability to trap the induced tribocharges increased upon the addition of nanowires to the PVDF matrix. The enhanced surface-charge potential and the charge-trapping capabilities of the PVDF-AgNW composite nanofibres significantly enhanced the TENG output performances. Moreover, the PVDF-AgNW composite nanofibres also exhibited excellent mechanical stability.43
A nanocomposite material system having a superior surface charge density as a triboelectric active material was reported. The nanocomposite material consisted of a high dielectric ceramic material of barium titanate together with a ferroelectric copolymer matrix, poly (vinylidene fluoride-co-trifluoro ethylene), showing great charge-trapping capability, electrically manipulated polarization and strong triboelectric charge-transfer characteristics. A boosted power-generating performance was achieved to 1130 V output voltage and 1.5 mA output current by this ferroelectric composite-based TENG under 6 kgf pushing force at 5 Hz.44
In order to overcome the interference of environmental factors on the performance of a TENG in practical applications, nanocomposite materials were developed to enhance the wear resistance and high-temperature resistance. A TENG was designed to deal with harsh environments and demonstrated a new wear-resistant triboelectric material by hybridizing a nanocomposite as the triboelectric layer. The layer was directly used for key wear-resistant parts. The nano-sized aluminum balls directly collided up and down between the two PTFE films enhancing the triboelectricity output. The results showed that the nanocomposite had a good wear resistance with a mean dynamic friction coefficient of about 0.69 μm at a low-friction of 8.1 N and room temperature. It had excellent high-temperature tolerance (temperature range of −30 °C to 550 °C), wear-resisting ability and high hardness (Rockwell Hardness about 63 HRM), making it capable of being used as a key supporting part, such as in automobile brake pads. In addition, it was found that the TENG output was 221 V, 27.9 μA cm−2 and 33.4 μC cm−2.40
Direct-current output is another practical requirement. A conventional TENG converted frictional energy into electricity by producing alternating current triboelectricity, which was limited by a low current density and the need for rectification. A continuous direct-current with a maximum density of 106 A m−2 could be directly generated by a sliding Schottky nanocontact without the application of an external voltage. Also, this was experimentally proved by sliding a conductive-atomic force microscope tip on a thin film of molybdenum disulfide (MoS2). Finite element simulation revealed that the anomalously high current density could be attributed to the non-equilibrium carrier-transport phenomenon enhanced by the strong local electrical field (105–106 V m−2) at the conductive nanoscale tip. This study indicated that the rectifying Schottky barrier at the tip–sample interface played a critical role in efficient direct-current energy harvesting, which makes nanomaterials promising for efficient direct-current triboelectricity generation.45
Energy-harvesting technology may be considered an ultimate solution to replace batteries and to provide a long-term power supply for wireless sensor networks. The development of multifunctional nanomaterials has greatly promoted developments in this field. Energy generation is moving towards more efficient, flexible, wearable and lightweight applications, where multifunctional inorganic nanomaterials bring new candidate materials for the development of energy-harvesting applications.
2.2 Energy-conversion applications
In recent years, perovskites have emerged as promising materials for low-cost, flexible and highly efficient solar cells.47 Despite their processing advantages, before the technology can be commercialized, the poor stability of the perovskite materials with regard to humidity, heat, light and oxygen has to be overcome.47 CsYbI3 cubic nanocrystals have exhibited strong excitation-independent emission and a high photoluminescence quantum yields of 58%.48 Perovskite solar cells (PSCs) have developed rapidly over the past few years, and the power conversion efficiency of PSCs now exceeds 20%. Such high performance can be attributed to the unique properties of perovskite materials, such as their high absorption over the visible range and long diffusion length.49 Due to the different diffusion lengths of holes and electrons, the electron-transporting materials (ETMs) used in PSCs play a critical role in the performance of PSCs. ZnO materials have a similar energy band position and physical properties to TiO2 but with much higher electron mobility, which can potentially improve the electron-transport efficiency and reduce the recombination loss as an ETM.49,50 First of all, ZnO has a very high transmittance in the visible spectrum and more importantly, is low in cost. Second, ZnO is easy to crystallize and to be doped. At the same time, the layered structure of the ZnO crystal leads to different growth rates along different directions. As a result, various ZnO nanostructures can be easily fabricated.49 High-efficiency methylammonium (MA) and Cs co-alloyed formamidinium (FA) triple cation perovskite-based ZnO solar cells have been reported. ZnO solar cells with a high crystallization multiple cation perovskite absorber showed a high efficiency of over 20%.51
A highly crystalline and compositionally photostable material, namely [HC(NH2)2]0.83Cs0.17Pb(I0.6Br0.4)3, with an optical band gap of about 1.74 eV, was reported.52 The fabricated perovskite cells reached an open-circuit voltage of 1.2 V and a power conversion efficiency of 14.7% on 0.715 cm2 cells. By combining these perovskite cells with a 19%-efficient silicon cell, the feasibility of 25%-efficient four-terminal tandem cells could be achieved. The incorporation of rubidium cations into the PSC improved the PV performance.53 The cells achieved stabilized efficiencies of up to 21.6% (average value, 20.2%) on small areas (and a stabilized 19.0% on a cell 0.5 cm2 in area) as well as an electroluminescence of 3.8%.53 Perovskite films based on CH3NH3PbI3 underwent rapid degradation when exposed to oxygen and light.54 Fast oxygen diffusion into CH3NH3PbI3 films was accompanied by the photo-induced formation of highly reactive superoxide species. Perovskite films composed of small crystallites show higher yields of superoxide and a lower stability. Ab initio simulations indicated that iodide vacancies are the preferred sites in mediating the photo-induced formation of superoxide species from oxygen.54 A N2 blow-drying method enhanced the hole-conducting effect of NiO in printable PSC.51 The best performing device demonstrated a remarkable PV performance with a short-circuit current density of 22.38 mA cm−2, an open circuit voltage of 0.97 V and a fill factor of 0.50, corresponding to a photoconversion efficiency of 10.83%.55 A solar cell based on this Sb2S3 film achieved a power conversion efficiency of 4.3%, which is the highest value reported in solution-processed planar heterojunction solar cells based on Sb2S3 films.56 The partial substitution of FA+ by MA+ can give the α phase under low temperature, which is beneficial to perovskite stability. Moreover, the stability against moisture and light illumination can be remarkably enhanced by partly substituting FA+ by a caesium cation (Cs+); the stability could be further improved with a mixture of three cations: Cs+/MA+/FA+.47 Highly efficient inverted solar cells based on perovskite were grown on nanostructures mediated by CuSCN.57 Three different CuSCN nanostructures were first applied to inverted heterojunction PSC as p-type inorganic hole-transport layers using a moderate electrodeposition method at room temperature. It was revealed that the crystal structure and the thickness of the CuSCN layer could dramatically regulate the morphology and the crystal orientation behaviour of perovskite absorbing layers, which will further have a significant influence on the PV device performance.
A bication lead iodide 2D perovskite component could stabilize the inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells.58 α-CsPbI3, with the most suitable band gap for tandem solar cell applications, faces issues around the phase instability under ambient conditions. A small amount of 2D EDAPbI4 perovskite containing the ethylenediamine (EDA) cation could stabilize α-CsPbI3 to avoid an undesirable formation of the nonperovskite δ phase.58 Stable high-efficiency 2D PSC could be achieved by caesium doping.59 Cs+-Doped 2D (BA)2(MA)3Pb4I13 PSC could achieve a power conversion efficiency (PCE) as high as 13.7%, the highest among the reported 2D devices, together with excellent humidity resistance.60 Cation-transmutation to design stable inorganic Pb-free halide perovskites for solar cells could address the key issue about the poor device stabilities associated with their intrinsic material instability. The idea here is to convert two divalent Pb2+ into one monovalent M+ and one trivalent M3+, forming a rich class of quaternary halides in a double-perovskite structure.61 The ionic defects at the surfaces and grain boundaries of organic–inorganic halide perovskite films are detrimental to both the efficiency and stability of perovskite solar cells. Defect passivation was consequently developed by using quaternary ammonium halide anions and cations in hybrid perovskite solar cells.62 An inorganic interlayer of spinel cobaltite oxides (Co3O4) could greatly enhance the carbon-based PSC performance by suppressing charge recombination and extracting holes efficiently. PCE is restricted by the charge-carrier transport and recombination processes at the carbon–perovskite interface. Devices realized with the screen-printed Co3O4 interlayer exhibited an 18% higher PCE of 13.27% as compared to standard carbon devices.63 A monolithic perovskite/Cu(In,Ga)Se2 tandem solar cell achieved an efficiency of 22.43%. Non-encapsulated devices under ambient conditions maintained 88% of their initial efficiency after 500 h of aging under continuous 1-sun illumination.64 A major factor limiting the performance of nanostructured CuInS2 photovoltaic devices is the current density, highlighting the poor charge-carrier transport in CuInS2 nanoparticle films.65 ZnS is typically chosen as a shell material for CuInS2 core passivation, which leads to a significant enhancement of the photoluminescence quantum yield from the CuInS2 nanoparticles. Replacing the typical divalent Zn cation surface termination with a monovalent Ag cation leads to small improvements in charge-carrier transport through nanostructured films.65 This surface termination intentionally introduces lower energy electronic states directly at the surface of the CuInS2 nanoparticles, reducing charge-carrier confinement and thus increasing the charge-carrier mobility between nanoparticles.65
Halide perovskites, with a typical structure of ABX3 (A = CH3NH3+, CH(NH2)2+, Cs+; B = Pb2+, Sn2+; X = Cl−, Br−, I−), have been demonstrated to be promising.66 In particular, inorganic halide perovskites are attracting increasing attention because of their higher stability towards moisture, light and heat. Inorganic halide perovskites consist of inorganic A site cations, such as Cs+.66 Inorganic halide perovskite nanomaterials provide a controllable morphology, tunable optoelectronic properties and improved quantum efficiency, as shown in Fig. 5.66
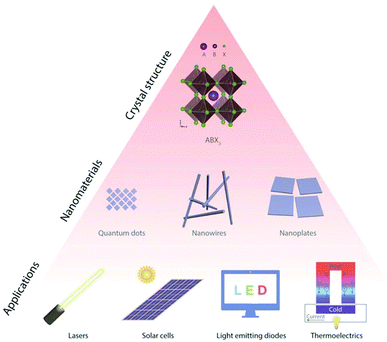 | ||
| Fig. 5 Schematic showing the design of some typical inorganic halide perovskite crystal structures (ABX3) to various nanostructures, and then their integration in different applications. This figure was reproduced from ref. 66 with permission from John Wiley and Sons, copyright 2018. | ||
Core–shell ZnO@SnO2 nanoparticles were synthesized, and used as novel electron-transport materials in perovskite solar cells. Thanks to the high electron mobility of core–shell ZnO@SnO2 nanoparticles, the PCE of solar cells reached 14.35%.67 TiO2–SiO2 core–shell nanostructured anti-reflective coatings were introduced in Cu(In,Ga)Se2 solar cells, and could prolong the optical path length by a scattering effect or by the formation of a refractive index gradient. The suppression of reflectance resulted in an improved PCE from 6.32% to 7.00% after applying the TiO2–SiO2 core–shell nanostructures.68 The piezo-phototronic effect could be effectively applied to improve the relative conversion efficiency of a flexible solar cell based on n-ZnO/p-SnS core–shell nanowire array.69 The efficiencies of quantum dot solar cells are limited by the high density of trap states caused by lattice imperfections on the quantum dot surface. PbS–CdS core–shell quantum dots were adapted to passivate the trap states. The results demonstrated that this may be caused by the improved passivation of the PbS surface by the CdS shell, leading to a lower electron trap density.70 A nanostructured WO3–TiO2 core–shell electron-transporting material was applied to perovskite solar cells, leading to an observed improved performance, which may be due to the suppressed charge recombination at the WO3/perovskite interface, faster charge separation and extended diffusion length of the charge carriers.71 PbSe/PbS core/shell quantum dots were used in solar cells, showing a short circuit current enhancement without the loss of open circuit voltage by shell thickness. PbS shell formation on the PbSe core mitigated the trade-off relationship between the open circuit voltage and the short circuit current density.72
Multimetallic nanosheets with single or few atoms thickness, are attracting extensive research attention because they display remarkable advantages over their bulk counterparts, including high electron mobility, unsaturated surface coordination, a high aspect ratio and distinctive physical, chemical and electronic properties.77 A non-Pt cathode catalyst was shown to be capable of simultaneously reducing both O2 and H2O2.78 The applicability of this unique catalyst was demonstrated by employing it in a fuel cell running on H2/CO and O2/H2O2.78 Ultrathin PdCu alloy nanosheets were used as a highly efficient electrocatalyst for formic acid oxidation.79 The excellent catalytic activity of PdCu nanosheets towards formic acid oxidation could be attributed to their ultrathin morphology, unique electronic structure and synergistic effect between Pd and Cu.79 A carbon-nanotube-supported nickel catalyst was prepared for fuel cells. The functionalized redox nanomaterial exhibited reversible electrocatalytic activity for the H2/2H+ interconversion from pH 0 to 9, with a catalytic preference for H2 oxidation at all pH values.80Fig. 6a shows a representation of the membrane-less fuel cell set-up using a 5 mm thick Teflon chamber instead of a classic proton-exchange membrane. The hybrid fuel cell was operated at 25 °C with humidified streams of H2 (atmospheric pressure) and with air feeding the anode and the cathode, respectively. Fig. 6b displays the polarization and power curves, as determined from successive galvanostatic discharges measured for 30 s.80
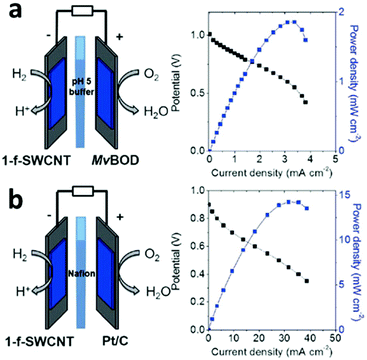 | ||
| Fig. 6 Representation of a H2/air fuel cell and polarization (black) and power (blue) curves for (a) single-walled carbon nanotubes|Myrothecium verrucaria (MvBOD)-carbon nanotubes (f-MWCNT) (phosphate buffer pH 5, 25 °C) and (b) single-walled carbon nanotubes|Pt/C (PEMFC, 60 °C, Nafion membrane). This figure was reproduced from ref. 80 with permission from John Wiley and Sons, copyright 2017. | ||
Pt–C core–shell was grown on carbon nanofibres, and then used as a cathode catalyst for the ORR in proton exchange membrane fuel cells. The synthesized Pt–C core–shell catalyst almost fully maintained the unit cell performance over 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 stability test cycles with negligible cell voltage loss. The excellent performance may be due to the robustness of the carbon shells that secured the Pt nanoparticles.81 IrNi-PdIr/C was prepared via a galvanic replacement reaction. It exhibited enhanced hydrogen oxidation activity in alkaline anion exchange membrane fuel cells. XRD and XPS analyses suggested that the enhanced activity could be attributed to the weakening of the hydrogen binding to the PdIr overlayers induced by the IrNi core.82 Pd-PtCu core–shell nanoplates exhibited substantially improved electrocatalytic performance for oxygen reduction and methanol oxidation reactions in fuel cells.83 A Pt-core and RhxSy-shell catalyst was synthesized for HER and HOR in a H2–Br2 fuel cell. The results showed that the catalysts with a core–shell structure had a higher active surface area compared to a commercial catalyst. Compared to the platinum catalyst, the core–shell catalysts showed more stable performance in fuel cell cycling tests.84
000 stability test cycles with negligible cell voltage loss. The excellent performance may be due to the robustness of the carbon shells that secured the Pt nanoparticles.81 IrNi-PdIr/C was prepared via a galvanic replacement reaction. It exhibited enhanced hydrogen oxidation activity in alkaline anion exchange membrane fuel cells. XRD and XPS analyses suggested that the enhanced activity could be attributed to the weakening of the hydrogen binding to the PdIr overlayers induced by the IrNi core.82 Pd-PtCu core–shell nanoplates exhibited substantially improved electrocatalytic performance for oxygen reduction and methanol oxidation reactions in fuel cells.83 A Pt-core and RhxSy-shell catalyst was synthesized for HER and HOR in a H2–Br2 fuel cell. The results showed that the catalysts with a core–shell structure had a higher active surface area compared to a commercial catalyst. Compared to the platinum catalyst, the core–shell catalysts showed more stable performance in fuel cell cycling tests.84
Carbon nanomaterials are promising metal-free catalysts for energy conversion and storage. Carbon nanomaterials are used as efficient photo-/electrocatalysts to facilitate the critical chemical reactions in clean and sustainable energy technologies.91 These reactions include the ORR in fuel cells, the OER in metal–air batteries, the iodine reduction reaction in dye-sensitized solar cells, the HER in water splitting and the carbon dioxide reduction in artificial photosynthesis.91 For carbon materials, the methods of tuning the electronic properties for creating active centres are illustrated in Fig. 7.91 The carbon atoms can be substituted by p-block elements, such as N, P, Si and B, or bonded with the heteroatoms, such as O, S, Se, F, Cl, Br and I.91 Intrinsic defects existing in carbon nanomaterials could induce charge transfer and a density of state change, thus generating active centres. The common methods of tuning the electronic properties of carbon materials for creating active centres are shown in Fig. 7.91
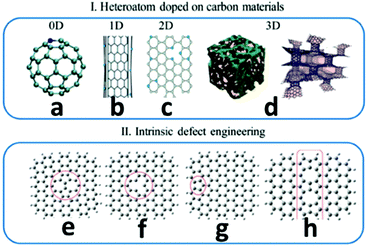 | ||
| Fig. 7 Methods of tuning the electronic properties of carbon materials for creating active centres. (I) Heteroatom-doping on carbon materials, including: (a) 0D fullerene, (b) 1D CNTs, (c) 2D graphene, and (d) 3D hybrid hierarchical structures. (II) Intrinsic defect engineering on graphene, including: (e) Stone–Wales defects, (f) a vacancy, (g) an edge defect with a nonhexagonal carbon ring and (h) a line defect-558 carbon-ring grain boundary. This figure was reproduced from ref. 91 with permission from John Wiley and Sons, copyright 2019. | ||
Atomically dispersed Zn–N–C nanomaterials are promising platinum-free catalysts for the ORR. However, the fabrication of Zn–N–C catalysts with a high Zn loading remains a formidable challenge owing to the high volatility of the Zn precursor during high-temperature annealing.92 Luckily, an atomically dispersed Zn–N–C catalyst with an ultrahigh Zn loading of 9.33 wt% could be successfully prepared by simply adopting a very low annealing rate.92 Electrochemical experiments showed that the atomically dispersed Zn–N–C catalyst exhibited not only comparable ORR activity to that of a Fe–N–C catalyst in both acidic and alkaline media, but also better durability than the Fe–N–C catalyst.92 Carbon-rich nanomaterials are fascinating hydrogen and oxygen electrocatalysts.93 As illustrated in Fig. 8, the reversible water dissociation processes comprise four essential half reactions, that is, ORR, HER, OER and the hydrogen oxidation reaction (HOR).93–97 For the ORR, nitrogen-doped carbon and atomic-metal-doped carbons have demonstrated a higher ORR activity than the Pt/C in alkaline solutions. For the HER, the HER activity of electrocatalysts is dominated by the adsorption free energy of the hydrogen intermediates in acidic solutions.94 For the OER, among the previously reported electrocatalysts and noble-metal-based catalysts (IrO2, Ir/C and RuO2), MOFs manifest the highest performance in alkaline media. In order to further reduce the cost and improve the energy conversion efficiency, it is a highly promising strategy to develop multifunctional electrocatalysts.93 Ultrathin PtPd-based nanorings with abundant step atoms enhance oxygen catalysis. Here, PtPdCo showed the highest ORR mass and specific activities of 3.58 A mg−1 and 4.90 mA cm−2 at 0.9 V versus RHE, i.e. 23.9 and 24.5-fold times larger than those of commercial Pt/C in alkaline electrolyte, respectively.98 A porous silicon and nitrogen co-doped carbon (SiNC) nanomaterial prepared by a facile pyrolyzation was developed as a metal-free multifunctional electrocatalyst.88 CO2-to-CO conversion and the OER partial current density under neutral conditions were enhanced by two orders of magnitude in the Tafel regime on SiNC relative to single-doped comparisons beyond their specific area gap. The photovoltaic-driven CO2 splitting device with SiNC electrodes imitating photosynthesis yielded an overall solar-to-chemical efficiency of 12.5% at only 650 mV overpotential.88
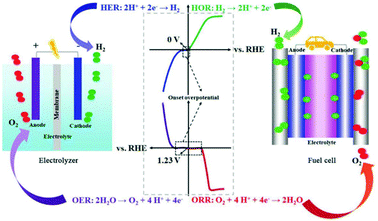 | ||
| Fig. 8 Schematic illustration of the water-splitting electrolyzer (left) and fuel-cell (right) devices as well as the corresponding electrochemical reactions. This figure was reproduced from ref. 93 with permission from John Wiley and Sons, copyright 2018. | ||
Controlling the MoS2 shell numbers can tune the tensile surface strain on Co9S8–MoS2 core–shell nanocrystals for boosting the hydrogen evolution reaction activity. It was found that the tensile surface strain could be precisely tuned from 3.5% to 0%. Here, Co9S8–MoS2 core–shell nanocrystals showed an overpotential of only 97 mV and a Tafel slope of 71 mV dec−1.99 CoP-PS exhibited a noticeable HER activity of approximately 80 mV with excellent durability.100 PtPb-Pt core–shell nanoplates boosted oxygen reduction catalysis. The nanoplates reported also showed high electrocatalytic activity and stability towards anodic fuel cell reactions, such as the methanol oxidation reaction and ethanol oxidation reaction.101
2.3 Energy storage applications
The development of renewable energy storage devices is one of the most promising ways to address the current energy crisis, along with global environmental concerns. The exploration of suitable active materials is the key factor to the construction of highly efficient, highly stable, low cost and environmentally friendly energy storage devices.102 Nanomaterials with a large specific surface area were proposed to guide future research towards closing the gap between the achieved and theoretical capacitance, without limiting the loading mass.103 Electrochemical energy storage devices, such as supercapacitors and batteries, are critical for enabling renewable yet intermittent sources of energy, such as solar and wind.104 Charge is stored only at the surfaces in supercapacitors, which are not limited by diffusion processes, allowing high power to be achieved. Similarly, because charging and discharging do not involve a bulk-phase transformation, supercapacitors are much more reversible and have a longer cycle life.105 Batteries store charge typically through redox reactions in the bulk phase of electrode materials, which leads to a higher energy density but lower power performance compared to supercapacitors. The demand for high-performance rechargeable batteries has become so tangible and ubiquitous in recent years that its numerous requirements and functions have nearly risen to the status of common knowledge.106,107Novel colloidal electrode materials were developed by Xue's group, and could deliver a higher specific capacitance than electrical double-layer capacitors and traditional pseudocapacitors.110–117 The electrode materials in colloidal supercapacitors were formed in situ, transformed from commercial rare earth and transition metal salts in alkaline electrolyte by chemical and electrochemical assisted coprecipitation.110 In these colloidal supercapacitors, multiple-electron faradaic redox reactions can be utilized, which can deliver ultrahigh specific capacitance, often larger than one-electron capacitance. The multiple-valence metal cations used in these designed colloidal supercapacitors mainly include Ce3+, Yb3+, Er3+, Fe3+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Sn2+ and Sn4+.114,115,118–125 The colloidal supercapacitors can serve as promising next-generation high-performance candidates.110
A novel and creative in situ electrochemical activation method to transform vanadium ions into a highly electroactive colloidal cathode in KOH solution under an electric field was designed. The vanadium-based colloids//activated carbon asymmetric supercapacitor displayed a high energy density of 50.4 W h kg−1 at a power density of 250 W kg−1, which was higher than most reported vanadium-based supercapacitors.126 Highly electroactive Mn7O13·5H2O colloids were formed in situ by an electric-field-assisted chemical coprecipitation in KOH solution. The highly efficient faradaic redox reactions were confirmed in pseudocapacitors, which could deliver a high specific capacitance of 2518 F g−1 based on active Mn cations at a current density of 5 A g−1. The present results showed that Mn cations in the designed system could lead to two-electron faradaic reactions.121 The active central ions of Fe3+ in Fe(NO3)3·9H2O could be fixed on the electrodes by the surrounding ligands (OH− and NO3−) and then could be in situ transformed into colloidal Fe4NO3(OH)11 and α-FeOOH. The electrochemical results indicated that the current proposed colloidal pseudocapacitor system enabled the highly efficient utilization of electroactive central Fe3+ ions, showing a high energy density of 58.4 W h kg−1 at a power density of 8.4 kW kg−1.119 Water-soluble CoCl2 electrodes showed the reversible redox reaction of Co2+ ↔ Co3+ ↔ Co4+ on the electrode and delivered a very high specific pseudocapacitance of about 1962 F g−1. Commercial CoCl2 salt was used directly as pseudocapacitor electrodes in an aqueous electrolyte, thus neglecting the complex synthesis procedures.116 Highly electroactive Ni-based colloidal electrode materials have also been synthesized by the in situ electrochemical activation of a NiCl2 electrode. The highest specific capacitance of the activated Ni-based electrodes was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 286 F g−1 at a current density of 3 A g−1, indicating that a three-electron faradaic redox reaction (Ni3+ ↔ Ni) occurred. Upon potential cycling and constant potential activation, a decrease in the charge-transfer resistance was found. The activation and utilization of multiple-electron reactions was shown to be an efficient route to increase the energy density of supercapacitors.123 Water-soluble CuCl2 electrodes showed a fast and reversible redox reaction of Cu2+ ↔ Cu+ and exhibited a very high specific pseudocapacitance of about 5442 F g−1. It was identified that the Cu2+ was responsible for achieving this superior value. The chemical and crystallization transformation of the CuCl2 electrode was presented.124 An in situ crystallization method for tin chloride salt pseudocapacitors was developed. After undergoing coupled chemical/electrochemical crystallization and faradaic redox reactions, highly active SnO/Sn colloids were in situ crystallized within the carbon black matrix. Such an electrode configuration allowed a fast transfer of ions/electrons and efficient utilization of the active tin cations in the salt electrode. The SnCl4 electrode could deliver an ultrahigh specific capacitance of 1592 F g−1 at a current density of 1 A g−1 during the potential range of 0.42 V, which is the highest value reported.125
286 F g−1 at a current density of 3 A g−1, indicating that a three-electron faradaic redox reaction (Ni3+ ↔ Ni) occurred. Upon potential cycling and constant potential activation, a decrease in the charge-transfer resistance was found. The activation and utilization of multiple-electron reactions was shown to be an efficient route to increase the energy density of supercapacitors.123 Water-soluble CuCl2 electrodes showed a fast and reversible redox reaction of Cu2+ ↔ Cu+ and exhibited a very high specific pseudocapacitance of about 5442 F g−1. It was identified that the Cu2+ was responsible for achieving this superior value. The chemical and crystallization transformation of the CuCl2 electrode was presented.124 An in situ crystallization method for tin chloride salt pseudocapacitors was developed. After undergoing coupled chemical/electrochemical crystallization and faradaic redox reactions, highly active SnO/Sn colloids were in situ crystallized within the carbon black matrix. Such an electrode configuration allowed a fast transfer of ions/electrons and efficient utilization of the active tin cations in the salt electrode. The SnCl4 electrode could deliver an ultrahigh specific capacitance of 1592 F g−1 at a current density of 1 A g−1 during the potential range of 0.42 V, which is the highest value reported.125
Rare earth elements have also been applied in supercapacitors. An ultrahigh specific capacitance of 2060 F g−1 could be obtained by the direct use of commercial Ce(NO3)3 as an electrode material in KOH electrolyte without any additional processing. Ce3+/Ce4+ could deliver a high practical specific capacitance close to its theoretical value.118 A new ErCl3 alkaline aqueous pseudocapacitor system was demonstrated by designing a commercial ErCl3 salt electrode in alkaline aqueous electrolyte. The synthesis of the material occurred at the same spatial and temporal scale. Highly electroactive ErOOH colloids were in situ crystallized via an electric-field-assisted chemical coprecipitation of ErCl3 in KOH aqueous electrolyte. These ErOOH colloids absorbed by carbon black and a PVDF matrix were highly redox-reactive with a high cation utilization ratio of 86% and a specific capacitance value of 1811 F g−1, exceeding the one-electron redox theoretical capacitance (Er3+ ↔ Er2+).114 The electrochemical performance and fabrication scheme are shown in Fig. 9. YbCl3 pseudocapacitor electrodes were synthesized in alkaline electrolytes, showing a high cation utilization ratio. The electrochemical reactive YbOOH colloids were crystallized through chemical coprecipitation and faradaic redox reactions. The effect of the crystallization kinetics on the electrochemical performance of the YbCl3 pseudocapacitor was studied. The YbCl3 pseudocapacitor showed an ultrahigh specific capacitance of 2210 F g−1.115
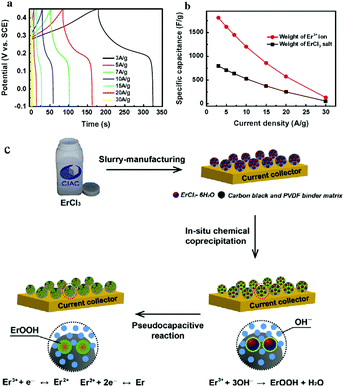 | ||
| Fig. 9 Electrochemical performance and fabrication scheme of the ErCl3 pseudocapacitor. (a) Charge/discharge curves (time versus potential) measured at various current densities and a potential range of 0.55 V. (b) Specific capacitance of the inorganic ErCl3 salt electrode versus the discharge current density at a potential window of 0.55 V based on the weight of ErCl3·6H2O salt and Er3+ ion. (c) Schematic drawing showing the in situ fabrication of the ErCl3 pseudocapacitor. First, the electrode was fabricated with the use of commercial ErCl3·6H2O salts by slurry-coating manufacturing. This figure was reproduced from ref. 114 with permission from Elsevier, copyright 2014. | ||
Crystalline-amorphous Fe2O3−δ core–shell architectures have also been successfully prepared. The core–shell architecture delivered a large capacitance of 701 F g−1 at 1 A g−1, which was almost double the capacitance of the conventional Fe2O3−δ nanorods without an amorphous surface layer on graphene.127 Phosphorus-doped Ni(OH)2 rods as the core and MnO2 nanosheets as the shell were fabricated. The core–shell structure exhibited an ultrahigh areal capacitance of 5.75 F cm−2 and great cyclic stability without capacitance loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.128 Zinc–nickel-cobalt oxide –Ni(OH)2 nanowire arrays showed an ultrahigh specific capacitance of 2847.5 F cm−3.129 Core–shell structured Co3O4–NiCo2O4 electrodes were grown on flexible carbon fibres. The core–shell structures presented an excellent specific capacitance of 1450 F g−1 at 1 A g−1. The unique structures supplied more pathways for accelerating fast electron and ion transfer.130 A nickel–cobalt metallic core and nickel–cobalt layered double hydroxide shell was grown on carbon fibre cloth. The synthesized architecture showed a high capacitance of 2200 F g−1 and retained 98.8% of its initial capacitance after 5000 cycles.131 MnCo2O4–Ni(OH)2 core–shell flowers were reported, with the structure shown in Fig. S1.† The nanoflowers exhibited an ultrahigh specific capacitance of 2154 F g−1 at a current density of 5 A g−1.132
000 cycles.128 Zinc–nickel-cobalt oxide –Ni(OH)2 nanowire arrays showed an ultrahigh specific capacitance of 2847.5 F cm−3.129 Core–shell structured Co3O4–NiCo2O4 electrodes were grown on flexible carbon fibres. The core–shell structures presented an excellent specific capacitance of 1450 F g−1 at 1 A g−1. The unique structures supplied more pathways for accelerating fast electron and ion transfer.130 A nickel–cobalt metallic core and nickel–cobalt layered double hydroxide shell was grown on carbon fibre cloth. The synthesized architecture showed a high capacitance of 2200 F g−1 and retained 98.8% of its initial capacitance after 5000 cycles.131 MnCo2O4–Ni(OH)2 core–shell flowers were reported, with the structure shown in Fig. S1.† The nanoflowers exhibited an ultrahigh specific capacitance of 2154 F g−1 at a current density of 5 A g−1.132
The energy storage of the battery follows the ion insertion/extraction mechanism. Take the lithium-ion battery as an example: during the discharging process, the cathode material is oxidized, resulting in the extraction of lithium ions from the electrode bulk phase. Simultaneously, a reduction reaction occurs in the anode material, which causes the lithium ions in the electrolyte to form a metal or alloy. As for the charging process, the reversible reactions occur on both electrodes.137 Therefore, the functions of the electrode material, especially the electronic and ionic conductivity, determine the electrode reactions. Electrode materials with effective ion migration channels, high electronic conductivity and abundant reactive sites are promising to achieve high capacity. In addition, the insertion/extraction of ions will deform the structure of electrode materials, resulting in volume changes, or can even collapse the channels, leading to a decay of capacity.138
Previous studies have shown that reducing the size of materials to the nanoscale can expose more active sites, which is conducive to the electrode capacity approaching the theoretical value. Xue et al. investigated the effect of the size of the manganese dioxide electrode on the capacity of lithium-ion batteries. MnO2 contains two types of Mn centres: one is the effective Mn centre where the faradaic reactions can take place, the other cannot make contact with the electrolyte. The specific capacitance of MnO2 is closely dependent on the percentage of effective Mn centres in the faradaic reaction (XeffectiveMn). They calculated the relationship between XeffectiveMn and the particle size. Their results showed that as the particle size decreased to several nanometres, XeffectiveMn increased significantly. It is worth noting that when the MnO2 size was smaller than 1 nm, XeffectiveMn equalled 1, indicating that the specific capacitance of MnO2 should achieve its theoretical value. Moreover, the mechanism of charge storage in adsorption/desorption or insertion/extraction processes can be distinguished by calculations. As shown in Fig. 10b, 94.74% of the specific capacitance of α-MnO2 with a size of 2 nm arose from adsorption/desorption processes, and only 5.26% of the specific capacitance arose from the insertion/extraction process.139,140
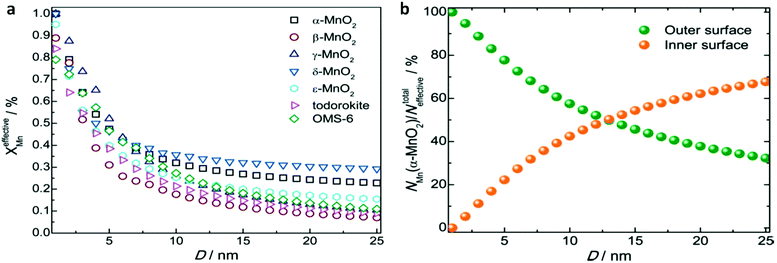 | ||
| Fig. 10 Size-dependent effective Mn centres in the faradaic charge storage of MnO2. (a) Size-dependent percentage of effective Mn centres of MnO2 polymorphs in the faradaic reaction. (b) Size-dependent percentage of effective Mn centres contributing to the adsorption/desorption and insertion/extraction processes of α-MnO2. This figure was reproduced from ref. 139 with permission from Royal Society of Chemistry, copyright 2016. | ||
Nanoscale substitutional solid-solution Mn1−xFexP compounds were synthesized as an anode for lithium-ion batteries. Enhanced electrochemical performance resulted from the in situ-generated nanocomposite of the Li–Mn–P alloying element and the Fe nano-network in combination with the surrounding amorphous lithium phosphide, which effectively buffered the accompanying volume variation, hindered the aggregation of the alloying element and ensured electron and ion transport. The Mn1−xFexP solid-solution phosphide electrodes exhibited a capacity of 360 mA h g−1 after 100 cycles at a high current density of 2 A g−1.144
A self-supported Cu3Si-Si@carbon@graphene (Cu3Si-SCG) nanocomposite anode was reported to mitigate the issues of the dramatic volume variation (>300%) during the lithiation/delithiation processes. The nanocomposite was composed of a Cu3Si-Si core and carbon shell with core/shell particles uniformly encapsulated by graphene nanosheets anchored directly on a Cu foil. The carbon shell, the highly elastic graphene nanosheet and the formed Cu3Si phase in Si served as buffer media to suppress the volume variation of Si during lithiation/delithiation processes and to facilitate the formation of a stable solid electrolyte interface (SEI) layer as well as to enable good transport kinetics. The optimized Cu3Si-SCG nanocomposite anode exhibited good rate performance and delivered a reversible capacity of 483 mA h g−1 (based on the total weight of Cu3Si-SCG) after 500 cycles with a capacity retention of about 80% at a high current density of 4 A g−1, rendering the nanocomposite a desirable anode candidate for high-performance LIBs.145
A foam-like FeS2 (F-FeS2) nanostructure was reported by combining solution combustion synthesis and solid-state sulfurization. The obtained F-FeS2 product was highly uniform and built from interconnected FeS2 nanoparticles (approximately 50 nm). The interconnected feature, small particle sizes and porous structure endowed the product with high electrical conductivity, good ion diffusion kinetics and a high inhibition capacity against volume expansion. As a result, it achieved a high capacity of 823 mA h g−1 at 0.1 A g−1, a good rate capability of 581 mA h g−1 at 5.0 A g−1 and a cycle ability of 97% retention after 80 cycles.150
VS4 microspheres were assembled from nano-units with different crystallinities via a facile template-free hydrothermal method to promote the electrochemical performance of 3D self-assembled nanoarchitectures. The results showed that the electrochemical performance of the VS4 microspheres as anode materials for sodium-ion batteries (SIBs) largely depended on their crystallinity. A VS4 electrode with the lowest crystallinity delivered a high reversible capacity of 412 mA h g−1 at 0.2 A g−1 after 230 cycles. The insertion mechanism was revealed within the selected voltage window of 0.50–3.00 V. Further analysis suggested that decreasing the crystallinity of the nano-units could dramatically enhance the pseudocapacitive behaviour of the VS4 microspheres, which took the main responsibility for the improvement of the sodium storage properties.151
Ti2Nb2O9 nanosheets with a tunnel structure can also be used as suitable anode materials for sodium-ion batteries. Ti2Nb2O9 nanosheets were synthesized by liquid exfoliation combined with topotactic dehydration, delivering a high reversible capacity of 250 mA h g−1 at 50 mA g−1 at a suitable average voltage of approximately 0.7 V. It was found that a low energy diffusion barrier, enlarged interlayer spacing and exceptional nanoporosity together gave rise to a high rate performance, as characterized by a pseudocapacitive behaviour. The observed high reversible capacity, excellent rate capability and good cyclability of Ti2Nb2O9 nanosheets make this material competitive when compared to other sodium-insertion anode materials.152
A 3D nitrogen-doped graphene/titanium nitride nanowires (3DNG/TiN) composite was reported as a freestanding electrode for Li–S batteries. The highly porous conductive graphene network provided efficient pathways for both electrons and ions. TiN nanowires attached on the graphene sheets had a strong chemical anchor effect on the polysulfides, as proved by its superior performance. As shown in Fig. 11, the 3DNG/TiN cathode exhibited an initial capacity of 1510 mA h g−1 and the capacity remained at 1267 mA h g−1 after 100 cycles at 0.5 C. Even at 5 C, a capacity of 676 mA h g−1 was reached. With a high sulfur loading of 9.6 mg cm−2, the 3DNG cathode achieved an ultrahigh areal capacity of 12.0 mA h cm−2 at a high current density of 8.03 mA cm−2. This proposed unique structure represents a bright prospect for simultaneously achieving a high energy density and high power density in Li–S batteries.155
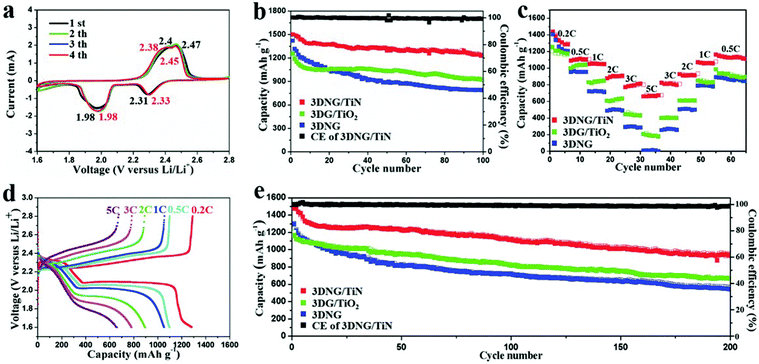 | ||
| Fig. 11 Electrochemical performances of 3DNG/TiN, 3DG/TiO2 and 3DNG cathodes. (a) CV profiles of the 3DNG/TiN at a scan rate of 0.1 mV s−1. (b) Cycling performance of the 3DNG/TiN, 3DG/TiO2 and 3DNG cathodes at 0.5 C for 100 cycles. (c) Rate performance of the 3DNG/TiN, 3DG/TiO2 and 3DNG cathodes. (d) Galvanostatic charge–discharge profiles of the 3DNG/TiN at different rates in a potential window from 1.6 to 2.8 V. (e) Cycling stability of the 3DNG/TiN, 3DG/TiO2 and 3DNG cathodes at 1 C for 200 cycles. This figure was reproduced from ref. 155 with permission from John Wiley and Sons, copyright 2018. | ||
Flexible batteries have become an indispensable component of many emerging devices, such as wearable, foldable electronics and sensors. Although various flexible batteries based on one-dimensional (1D) and two-dimensional (2D) platforms have been explored, developing a high energy density electrode with high structural integrity remains challenging. A flexible porous cathode for lithium–sulfur batteries was synthesized, consisting of reduced graphene oxide (rGO), graphene crumples (GCs) and sulfur powders. The electrode structures were tailored using GCs with different dimensions and functional features, which were critical to its robustness under mechanical deformation and electrolyte penetration into the battery components. The optimized rGO/GC/S composite ribbon cathodes delivered a high capacity of 524 mA h g−1 after 100 cycles at a current rate of 0.2 C. A shape-conformable battery prototype comprising an rGO/GC/S cathode and a lithium anode demonstrated stable discharge characteristics under repeated bending/flattening cycles. The LSB prototype supported by an elastomer presented a stable discharge behaviour with high mechanical robustness against an extension of up to 50%.156
A hollow Fe3C-N/C with a frogspawn-like architecture was proposed as a highly efficient sulfur host in this paper. Derived from a Prussian blue self-template, Fe3C-N/C possessed a metal-like Fe3C spawn core and the high conductivity of an N-doped carbon shell. This unique structure enabled a large surface area, fast e− and Li+ transport, as well as a large hollow space for volumetric expansion of the sulfur cathode. Moreover, with the N-doped carbon shell and the polar Fe3C core, the trapping and catalytic conversion of intermediate polysulfides were also facilitated. The strongly coupled interaction of polar Fe3C and polysulfides was confirmed by both theoretical calculations and the electrochemical performance. Specifically, the Fe3C-N/C/S electrode presented a high capacity of 1351 mA h g−1 at 0.1 C with Fe3C-N/C as an integrated sulfur host. In particular, the rate capability and cycling stability of the Fe3C-N/C/S electrode was outstanding. It displayed a high capacity of 792 mA h g−1 at 5 C and a low capacity decay rate of 0.08% per cycle at 0.5 C after 400 cycles.160
A core–shell architecture of dual-phase FeCo-based nanoparticles-heteroatom-doped carbon microspheres (FeCo-C MS) was synthesized via a two-step carbonization process from a reactive multifunctional core–double-shell template. With the advantages of a heterogeneous composition and architectural structure, the obtained FeCo-C MS was shown to be an excellent cathode catalyst for the Zn–air battery, and FeCo-C MS exhibited a high discharge voltage of 1.27 V, high specific capacity of 503 mA h g−1 and an energy density of 639 W h kg−1 (Fig. S2†).159
With the development of multifunctional inorganic nanomaterials, significant commercial and academic progress has been made on battery technologies. There is reason to believe that the invention of nanomaterials with novel structures will promote the better performance of batteries. Therefore, the use of efficient material screening methods using artificial intelligence is a growing trend in the development of energy storage applications.
2.4 Energy saving applications
Energy consumption by buildings accounts for a large proportion (about 40%) of the world-wide energy consumption, among which more than 50% is contributed by heating, ventilation and air conditioning.161–164 Smart windows in modern buildings can significantly decrease energy consumption via an efficient modulation of the light/heat passing through these windows.165 For smart windows, inorganic nanomaterials play an important role due to their unique properties. Smart windows can change their throughput of visual light and solar irradiation by using electrochromic materials characterized with a specific nanostructure, thereby avoiding excessive solar heating and controlling the heating, cooling, lighting and visual contact between indoors and outdoors.166As one of the most representative electrochromic materials, tungsten trioxide (WO3) has been extensively studied and has become recognized as a very promising material for smart window applications due to its advanced features, such as reversible colour switching from optical transparency to deep blue and good electrochemical stability and electrochromic performances, as compared organic electrochromic materials.165,167–170 Tunnelled phosphorus-doped WO3 films have been synthesized.170 The elaborate architecture of the tunnelled structure without the formation of any cracks could improve not only the switching speeds and colouration efficiency values by increasing the electroactive contact with the electrolyte, but also the cycling stability to 91.5% after 1000 cycles by effectively accommodating the charge diffusion and the expansion–contraction of the WO3 films. The accelerated effect of P doping on the WO3 films enhanced the electrical conductivity to favour the electrochromic performance by a higher degree of electron sharing. It is worth mentioning that with the development of multifunctional materials, smart window materials not only have the function of saving energy, but also have certain functions for storing energy. One device made of this material is the electrochromic energy storage window and this has become a new focus of research.171–173 Mesoporous WO3 film, which exhibited enhanced electrochromic properties, showed a high transparency at the bleached state, with an optical transmittance of 99.5% at a wavelength of 633 nm.165 The tiny WO3 nanoparticles were filled into the granular voids of the substrate and simultaneously decreased the surface roughness, which may be a contributing factor for the high transparency and good stability of the mesoporous film. It exhibited a fast colouration switching response, in which the colouration time was found to be 2.4 s, and the bleaching time was 1.2 s. In addition, a high colouration efficiency of 79.7 cm2 C−1 and large optical modulation of 75.6% at 633 nm were achieved. The mesoporous WO3 film also had the function of energy storage with a specific capacity of 75.3 mA h g−1.
Tungsten bronze (MxWO3, M = Li, Na, K, Rb, Cs, NH4) is a novel transparent conductive oxide with excellent visible transparency by selectively cutting off near infrared light and it showed near infrared shielding (NIR) induced by localized surface plasma resonance. It has potential applications in the field of energy saving smart window glasses.174–177 CsxWO3 nanorods were synthesized by controlling a variety of synthesis conditions to achieve a high light transmittance and high NIR shielding.174 According to thermal insulation testing, the temperature of the box covered with ordinary glass was as high as 77 °C after 1 h of illumination, while the temperature of the box covered with the CsxWO3 film glasses was only 50 °C.
NaxWO3 was obtained by annealing an amorphous non-stoichiometric WO3−x (0 < x < 1) film prepared on common commercial glass.177 NaWO3 had a high transmittance for visible light as well as excellent shielding for near-infrared light. In the process of simulating a house illuminated by sunlight, the temperature difference was at least 4.5 °C for 60 min relative to blank glass. Further, the application of tungsten bronze observably reduced energy consumption, decreased the emission of carbon dioxide and thereby improved the living environment. However, from the viewpoint of practical applications, the VIS light transmittance and UV light blocking properties of hexagonal tungsten bronzes also require further improvement owing to their relative narrow band gap (2.5–3 eV).178 Pt-Doped KxWO3 nanoparticles were synthesized.176 Trace Pt doping could contribute to the uniform growth of hexagonal KxWO3 nanorods and could significantly improve the NIR performance. In heat insulation property tests, a sample coated with the Pt-doped KxWO3 film had a temperature of only 40 °C after 40 min of irradiation with a 250 W infrared light, and the sample temperature of the blank glass and the PVA film was as high as 60 °C, demonstrating the excellent heat insulation of Pt-doped KxWO3. To address the problem where the smart window glass is easily contaminated by dust, organic and inorganic particulate matter and microbial growth, the K0.3WO3/Ag2O nanocomposites were synthesized with a self-cleaning function.175 The self-cleaning function was studied by monitoring the decomposition of rhodamine B (RhB) under visible light. After 2 h visible light irradiation, the photodegradation rate of RhB by the K0.3WO3 film was only 29%, while the photodegradation rate of the K0.3WO3/Ag2O film was as high as 74%. The photogenerated electrons of K0.3WO3 transfer to the conduction band of Ag2O and the holes of Ag2O migrate to the valence band of K0.3WO3. These well-separated photoelectrons and holes can further react with absorbed oxygen molecules and water molecules, generating ˙O2− and ˙OH. Finally, the obtained ˙O2− and ˙OH degrade the dye molecules.
ZnO is an n-type semiconductor with interesting optical, thermal and electrical characteristics, and a very rich defect chemistry. Such features, coupled with its unique flexibility in adopting a large variety of nanoscale morphologies, have made ZnO an attractive candidate for optoelectronic and gas sensing applications.179 A series of MxWO3/ZnO (M = K, Rb, NH4) composites was prepared to achieve heat insulation, air decontamination, harmful UV light blocking and high VIS light transmittance simultaneously.178 The combination of these two materials significantly improved the performance and increased the potential for tungsten bronze to become a commercial smart window material. A designed lithium-ion insertion-type material layer on a fluorine-doped tin oxide substrate with a TiO2 mesoporous nanotube array film was prepared. It had rapid Li-ion insertion kinetics without sacrificing the window transparency.180 Because of the rapid Li-ion insertion kinetics, including an enhanced pseudocapacitive effect and Li-ion diffusion coefficient, the device exhibited a high-rate capability over short galvanostatic charge/discharge times. At 1 A g−1, it completed the charge or discharge process within only 232 s and delivered a high, reversible and stable specific capacity of 60 mA h g−1.
Nanofluids are nanoparticle dispersions that can be dispersed in liquids to form a stable system. They do not affect the rheological properties of the system. Nanoparticles in nanofluids have a high surface area and therefore have more heat transfer surfaces than liquids.181,182 This leads to a high thermal conductivity for nanofluids and so they are widely used in heat-transfer applications. The use of nanofluids to heat buildings can reduce the size of the heat-transfer system, the accompanying pressure losses and the need for subsequent pumping power. This can reduce the energy consumption of a power plant and indirectly reduce environmental pollution.183
Refrigeration systems are one of the biggest reasons for the expanding pattern of energy consumption, with approximately 15% of the world's electricity consumed by refrigeration and air conditioning systems.15,184 Energy savings in the refrigeration system can be achieved by reducing the amount of refrigerant required and by reducing the wear characteristics of the compressor. The refrigerants and lubricants used in the system need to have thermal, mechanical and chemical functions, so they require the use of multifunctional materials. At the same time, the materials must maintain high stability and non-toxicity, which highly conform to inorganic materials. The addition of nanomaterials can provide a higher thermal conductivity for the refrigerant, thereby reducing the amount of refrigerant required for the refrigeration system. It can also provide higher lubricity for the lubricant, thereby reducing the wear characteristics of the compressor and ultimately resulting in energy savings.15,185 As a result, multifunctional inorganic nanomaterials are essential for energy saving applications. Boron nitride nanosheets possess high thermal conductivity and excellent insulation property. Boron nitride nanosheets nanofluids were fabricated and their effects on thermal conductivity enhancements were further investigated. The thermal conductivity of the boron nitride nanosheets nanofluids could reach 2.39 W m−1 K−1, which represent a 298% increase in comparison with water. By measuring the thermal conductivity from 25 °C to 50 °C, the temperature dependence of boron nitride nanosheets nanofluids was awaken, because the large size of boron nitride nanosheets led to insignificant Brownian motion.185
Lubrication is defined as a process or technique utilized to reduce friction and wear between two surfaces in proximity and moving relative to one another by interposing a substance called a lubricant.186 The use of lubricants in mechanical systems with a good tribology characteristic will minimize the wear and friction, and thus make it possible to reduce the power consumption. In the presence of a lubricant, a slippery film is formed, which substantially reduces friction, wear and tear between mating surfaces. Apart from the reduction of friction and wear, the other major functions of lubricants are to improve cooling and heat transfer. In order to serve these purposes, lubricants should possess certain characteristics, such as high thermal and chemical stability, adequate viscosity and resistance to oxidation and corrosion. Nanofluids serving as a lubricating medium have been widely referred to as “nanolubricants” in the literature.
In addition to its marvellous properties, graphene, being a 2D layered structure, offers unique wear and friction properties that are rarely seen in conventional materials.186 Well-dispersed, clove-treated graphene nanoplatelet coolants were synthesized to achieve improved heat transfer and hydrodynamic properties. A clove-treated graphene nanoplatelet–water nanorefrigerant was tested in a horizontal tube heat exchanger to determine the heat transfer and hydrodynamic properties. A superior enhancement in the thermophysico properties of the clove-treated graphene nanoplatelet–water nanorefrigerant was found. A maximum thermal conductivity enhancement of 22.92% was attained for the sample containing 0.1 wt% of clove-treated graphene nanoplatelets at a fluid temperature of 45 °C. In addition, the dynamic viscosity and density of the clove-treated graphene nanoplatelet–water nanofluids were close to those for deionized water. There was a pronounced increase in the Nusselt number and convective heat transfer coefficient for the clove-treated graphene nanoplatelet–water nanofluid at a weight concentration of 0.1% with a maximum enhancement of approximately 18.69% and 37.54% when the Reynolds number was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927. More importantly, there was a negligible increase in the corresponding friction factor, with a value of 3.79%.187
927. More importantly, there was a negligible increase in the corresponding friction factor, with a value of 3.79%.187
Graphene oxide–TiO2 nanocomposites were synthesized by a facile solvothermal reaction between graphene oxide and tetrabutyl titanate.188 Test results showed that spherical TiO2 was successfully anchored on the graphene oxide nanosheets. The graphene oxide–TiO2 nanofluids exhibited better friction and wear properties than a lubricant with graphene oxide nanosheets, TiO2 nanoparcles and a mixture of graphene oxide and TiO2. The film thickness of the lubricant with graphene oxide–TiO2 was 28.07 nm, which was the largest in all the lubrication states.
Based on the experimental and test results, Fig. 12 presents the lubrication mechanism of graphene oxide–TiO2. The good dispersion stability and hydrophilicity of graphene oxide–TiO2 made it possible to lubricate in flakes, which means it can more efficiently enter the gaps between the rollers and strips. In addition, the presence of graphene oxide–TiO2 at the rough peak can avoid direct contact between the roll and the strip, and the TiO2 nanoparticles anchored on the graphene oxide nanosheet can only be deformed without rolling at will, ensuring the stability of the film formation. Finally, the increase in defects in the graphene oxide–TiO2 nanosheets indicated that the graphene oxide–TiO2 nanocomposites may be worn or torn into smaller particles during the lubrication process.
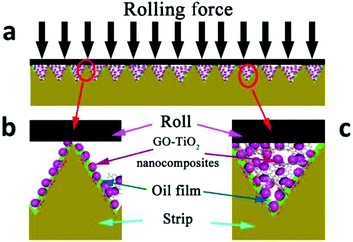 | ||
| Fig. 12 Different views of graphene oxide–TiO2 nanofluids rolling lubrication mechanism described in the reference. (a) Overall schematic, (b) rough peak view, (c) rough valley view. This figure was reproduced from ref. 188 with permission from Elsevier, copyright 2018. | ||
Enhanced tribological properties of a mineral base oil containing an antifriction nano-additive based on reduced graphene oxide nanosheets were reported. The graphene oxide nanosheets exhibited no clear acute toxicity of industrial grade. Furthermore, the author proposed a workflow for the evaluation of reduced graphene oxide-based nanolubricant for human and environmental toxicity considering different steps of the material value chain and different reduced graphene oxide dispersions.189 The stability of a nanolubricant is important to affirm the best performance in an automotive air conditioning system and to avoid system problems after long runs, such as clogging or sedimentation. SiO2/PAG nanolubricants were successfully synthesized by a sonication method. The performance parameters and energy savings of an automotive air conditioning system using SiO2/PAG nanolubricants were compared with PAG lubricants. It was found that the condenser pressure and the pressure ratio of the automotive air conditioning system decreased to an average of 10.8% and 5.6%, respectively. The volumetric heat absorb increased by up to 3% and the coefficient of performance increased by an average of 21%. The compressor work and power consumption of the automotive air conditioning system were reduced by 16.5% and 4%, respectively.190 The purpose of the sonication stage was to break the agglomeration and to provide a uniform nanoparticle dispersion. The coolant side pressure drop, as well as pumping power, could be reduced by using hybrid nanofluids.191 The maximum pumping power reduction was with Al2O3 + MWCNT (11.2%) and minimum with Al2O3 + Ag (9.6%). Furthermore, the payback period was considerably higher with the use of the hybrid nanofluid. Al2O3 + Ag showed a maximum of 247 years, while Al2O3 + TiO2 showed a minimum of 9.8 years. The payback period could be reduced by reducing the nanoparticle cost and increasing the nanofluid stability. This demonstrated the great potential of nanofluids in industrial applications.
To sum up, electrochromic materials and nanofluids are promising materials for energy saving applications. Electrochromic materials are the main materials for smart window applications and require high light transmission, reversible colour conversion, high chemical and electrochemical stability, and excellent near-infrared shielding properties. Furthermore, according to the additional functions of the device, the material also needs to have additional functions. For example, energy storage smart windows require the material to have an energy storage function, while the self-cleaning smart window requires the material to have a catalytic function. Nanofluids are mainly nanorefrigerants and nanolubricants. The addition of inorganic nanoparticles to traditional refrigerants and lubricants can significantly improve their thermal and lubricating properties. This is related to the size effect of the nanomaterials, where their large surface areas provide more heat transfer surface, and the addition of the nanoparticles thickening the film of the lubricant on the surface of the contact layer greatly improves the lubricating effect of the lubricant. Multifunctional inorganic materials provide a rich selection for smart window applications and nanorefrigerants and nanolubricants. Although the high performance of multifunctional inorganic materials is important, in energy saving applications, the price and toxicity of materials and the ease of material recovery should also be given priority.
2.5 Energy transmission applications
The development of reliable and environmentally friendly approaches for energy applications is one of the key challenges that modern society is facing.14 The large amount of greenhouse gases produced by burning fossil fuels are detrimental to global warming and climate change. The substitution of fossil fuels with environmentally friendly and renewable energy sources is therefore a promising way to reduce greenhouse gas emissions.192 A major challenge, however, is that such renewable energy sources, such as solar, wind and hydro energy sources, are often located far from densely populated areas, which means that electricity generation from these distant renewable energy sources must be combined with cost-effective energy transmission systems.11 Multifunctional inorganic nanomaterials are an important class of materials with significant potential for energy transmission applications, mainly focused on superconductors and smart grids.8 Since the discovery of superconductivity, it has been the dream of researchers to realize a room temperature superconductor.193 Recent experimental advances on inorganic nanomaterials have unveiled a range of exotic physical and electronic phenomena for superconductors, such as a reduced superconducting transition temperature upon reducing a sample thickness to the nanoscale and 2D superconductivity.194–199 In addition, the voltage levels have to increase to further reduce the energy transmission losses in the research and development application of smart grids, which presents a great challenge for high-voltage direct-current (HVDC) insulating materials. Recently, nanodielectrics (nanocomposites) based on an ultralow loading volume of metal oxide nanoparticles (<1 vol%) have been introduced as a potential material group for electrical insulation materials due to their lower charge mobility and direct-current conductivity.200–203High-quality superconductor/semiconductor hybrid structures, such as Al/InAs and V/InAs, are of interest for ongoing research in the fields of gateable Josephson junctions (JJs) and quantum-information-related research.212–214 Phase-dependent zero-bias conductance peaks measured by tunnelling spectroscopy at the end of Josephson junctions has been observed, as realized on a heterostructure consisting of aluminium on indium arsenide nanowires and its planar JJs were investigated as a function of the magnetic field, chemical potential and phase difference (Fig. 13). This work suggested that phase control could offer an additional tuning parameter to enter the topological regime that has not been explored so far.214
 | ||
| Fig. 13 Planar JJs phase dependence of the critical field. Third harmonic of the current I3ω(Vsd = 0) measured by the lock-in amplifier at zero bias as a function of B∥ and Φ, for different values of gate voltage V1 at (a) −116 mv, (b) −118.5 mv and (c) −120 mv. This figure was reproduced from ref. 183 with permission from Springer Nature, copyright 2019. | ||
The in situ growth of crystalline aluminium and niobium shells on indium arsenide nanowires by molecular beam epitaxy (MBE) was reported. The realization of new material combinations of Nb/InAs in addition to Al/InAs extended the temperature range and the critical fields possible for further research.215 Hybrid devices based on III–V nanowires with ex situ e-beam deposited vanadium contacts have previously been used for studies of Andreev bound states and superconducting quantum interference devices.216,217 A combined analysis of the crystal structural and electronic properties of vanadium deposited on InAs nanowires has already been presented. Nanoscale superconducting vanadium had a high out-of-plane critical field, far exceeding the bulk value and that of Al on InAs nanowires. The wide variety of grain sizes dominated the electrical behaviour of devices fabricated from those crystal growths. This work suggested better hybrid devices need further optimization of the entire crystalline and that there is a need to find a more suitable semiconductor to achieve epitaxially matched vanadium/semiconductor hybrids.211 In addition, low-dimension and small-size superconductors are also candidates for studying electrical transport in nanoscale systems due to their unique size-and shape-dependent properties. YBa2Cu3−xNixO7−δ (0.00 ≤ x ≤ 0.04) nanowires were prepared by an electrospinning technique. The temperature dependence of magnetization of these nanowires revealed a diamagnetic transition at temperatures close to the Tc, which was related to the superconductor transition in these compounds. The Tc values ranged between 70 K and 93.2 K for samples with x = 0.04 to x = 0.00, respectively, where the substitution of Cu with Ni resulted in a lower Tc. This behaviour could be interpreted as a distribution of the transition temperatures due to an inhomogeneous distribution of Ni atoms, granular effects and structural variations resulting from the synthesis process.218
2D superconductors have many interesting properties, such as the localization of electrons and Cooper pairs, transition-temperature oscillations caused by quantum size effects, excess conductivity originating from superconducting fluctuations, Berezinskii Kosterlitz Thouless (BKT) transitions and quantum phase transitions (QPTs) at zero temperature.219 With the continuous development of new preparation and stacking methods for ultrathin 2D materials with atomic layers, such as MBE, accompanied by new surface or interface reconstruction processes, mechanical exfoliation and methods for the production of field-effect devices, the emergence of van der Waals heterostructures of 2D materials has opened a new door for quantum materials.206,220–223 Novel physical properties, such as high temperature superconductivity, can be achieved by controlling the composition and stacking direction of different layered materials, or by changing the external electric field. Recently, signatures of tunable superconductivity in an ABC-trilayer graphene (TLG) and hexagonal boron nitride (hBN) moiré superlattice were reported. The electronic behaviour in the ABC-TLG/hBN superlattice depended sensitively on the interplay between the electron–electron interaction and the miniband bandwidth. Transitions from the candidate superconductor to a Mott insulator and metallic phases could be observed by varying the vertical displacement field (Fig. 14a). It was shown that ABC-TLG/hBN heterostructures offered attractive model systems in which to explore high Tc superconductivity's relationship to the Hubbard model.224 In addition, the heterostructures or hybrids of graphene and superconductors offered a very interesting platform to study mesoscopic superconductivity and the interplay of the quantum Hall effect with superconductivity. The direct growth of high-quality graphene/2D superconductor (nonlayered ultrathin α-Mo2C crystal) vertical heterostructures with uniformly well-aligned lattice orientation and strong interface coupling by chemical vapour deposition was reported. As shown in Fig. 14b and c, based on these strongly coupled high-quality heterostructures, highly transparent Josephson junction devices were realized, in which a clear magnetic-field-induced Fraunhofer pattern of the critical supercurrent was observed. This work provided a promising platform for future studies of exotic quantum transport behaviours, such as Majorna modes.225
 | ||
| Fig. 14 (a) Tunable electronic phases with the displacement field. As a function of D, the system can be tuned across four different electronic states, from left to right: superconducting, correlated resistive state, metal and correlated insulating state. This figure was reproduced from ref. 224 with permission from Springer Nature, copyright 2019. (b and c) Highly transparent Josephson junction device based on a CVD-grown graphene/2D α-Mo2C crystal heterostructure. This figure was reproduced from ref. 225 with permission from American Chimerical Society, copyright 2017. (d and e) NbSe2 temperature (d) and magnetic-field (e) dependent superconducting behaviours. This figure was reproduced from ref. 205 with permission from Royal Society of Chemistry, copyright 2017. | ||
The transition metal dichalcogenide niobium diselenide (NbSe2) is one of the most studied van der Waals materials that exhibits both CDW and superconductivity at low temperatures.195,205 There was a combined optical and electrical transport study performed on the many-body collective-order phase diagram of NbSe2 down to a thickness of one monolayer. Both the CDW and the superconducting phase were observed down to the monolayer limit. It was reported the superconducting transition temperature decreased on lowering the layer thickness, but the newly observed CDW transition temperature increased from 33 K in the bulk to 145 K in the monolayer, which could be understood to be a result of significantly enhanced electron–phonon interactions in 2D NbSe2. This work suggested van der Waals materials can provide an ideal platform for the investigations of CDWs and their relation with superconductivity in the 2D limit.9 High-quality NbSe2 nanoplates were synthesized by a single-step chemical vapour deposition and their temperature and magnetic-field dependent superconducting behaviours were investigated (Fig. 14d and e). The NbSe2 nanoplates showed the 2D characteristics of superconducting transitions and strong anisotropy with magnetic field orientation, thereby providing potential platforms for the exploration of new physics in nanoelectronic devices.205 In the future, the development of new technologies for the fabrication and characterization of 2D nanostructured superconductors will probably be in high demand.
As mentioned above, superconducting planar nanostructures are widely used in many applications. In contrast, 3D superconducting nanostructures, despite their potential in quantum information processing and nanoelectronics, have been addressed only in a few pioneering experiments.226–228 A comparative study of planar nanowires and free-standing 3D nanowires have been carried out by focused electron- and ion (Ga+)-beam induced deposition (FEBID and FIBID) using the precursor Nb(NMe2)3(N-t-Bu). Electrical transport measurements showed that FEBID nanowires were highly resistive following a variable-range-hopping behaviour. In contrast, FIBID planar nanowires became superconducting at 5 K. In addition, the critical temperature of free-standing 3D nanowires was as high as 11 K, which was close to the value of bulk NbC, which suggested that FIBID-NbC was a promising material for the development of 3D superconductivity, with applications in quantum information processing and nanoelectronics.204 The growth of 3D superconducting WC1−x hollow nanowires with the use of a He+ focused-ion-beam-microscope in combination with the W(CO)6 precursor was reported, and this became superconducting at 6.4 K and showed a large critical magnetic field and critical current density, resulting from their quasi-1D superconducting character. The results paved the way for future nanoelectronic devices based on 3D nanosuperconductors.228 The unique capability of 3D nanosuperconductors to achieve extreme in-plane and out-of-plane magnetic-field sensitivity allowed them to be used as a local probe for nanomagnets and nanosuperconductors. Hence, the exploitation of the three-dimensions in nanosuperconductivity could represent a paradigm shift for the development of next-generation electronic nanodevices with applications in advanced sensing and quantum computing.
Among these metal oxide particles, ZnO is relatively unique due to the possibility of tailoring this particular metal oxide into a broad range of particle sizes and shapes by a simple nonsurfactant aqueous precipitation under controlled synthesis conditions. High-purity, narrow-size-distribution ZnO particles with different sizes and morphologies were synthesized and a series of silanes with terminal alkyl groups of different lengths (methyl, octyl, and octadecyl groups) were used to modify the surface of the ZnO nanoparticles. These modified hydrophobic particles showed excellent dispersion/distribution in the LDPE matrix due to the high particle/polymer compatibility. The hierarchical flower-shaped ZnO particles with a nanosized porosity accessible to the LDPE chains showed a high interfacial surface area and additional electron traps for charge carriers at the polymer/particle interface, while the addition of 3 wt% ZnO nanoparticles with controlled functionality was sufficient to decrease the conductivity of pristine polyethylene by more than two orders of magnitude.200
In addition, MgO has become a research hotspot because of its relevance as an emerging inexpensive industrial nanoparticle material and due to its insulating properties. MgO/PP/POE nanocomposites were fabricated by melt blending. An examination of the electrical properties revealed that the DC electric breakdown strength and space charge suppression effect were remarkably improved by the introduction of surface-modified MgO nanoparticles. In addition, obvious enhancements in the tensile modulus and strength were obtained as a result of the synergistic toughening of the POE and MgO nanoparticles. All of these enhancements in the mechanical and electrical properties were closely related to the large interfacial zones and good adhesion properties between the nanoparticles and polymer matrix. These results suggested that MgO/PP/POE nanocomposites with enhanced mechanical and electrical properties have great potential to be used as recyclable insulation materials for high-voltage DC cables with large transmission capacities and high operating temperatures.229 Also, 70 nm hexagonal MgO nanoparticles with 18 units long hydrocarbon functional silsesquioxane coatings were synthesized. The lowest volume conductivity was about 7 × 10−16 S m−1 for 3 wt% surface-coated nanoparticles, which was a reduced conductivity of two orders of magnitude compared to pristine LDPE.203
In addition to metal oxide particles, carbon-based materials, such as fullerene (C60), have also been studied for their high electron affinity and low ionization energy for efficient electrical tree inhibition.231 In fact, the primary challenge in the processing of nanocomposites is achieving a homogeneous dispersion of the nanoparticles. The dispersion quality affects the interfaces between the organic and the inorganic components, which can determine the final properties of the nanocomposite.229,232 Besides, the search for high purity nanoparticles without conducting counterions on the particle surfaces is also very important for the development of ultralow electrical conductivity nanocomposites.233
3. Perspectives and conclusion
Recently, inorganic nanomaterials have been well developed for energy applications. Energy applications require materials with high electrical, optical, mechanical and thermal properties. In order to meet the requirements of these energy applications, multifunctional inorganic nanomaterials have been extensively studied. For each energy application, we illustrated the unique functions of inorganic nanomaterials to improve their performances. We highlighted the combination of functions of nanomaterials in a device. In most energy applications, the limited success so far may be traced back to the limitations set by the scaling relations between multifunctional inorganic nanomaterials and energy devices.Multifunctional inorganic nanomaterials play a key role in energy generation, energy conversion, energy storage, energy saving and energy transmission applications due to their unique properties, such as excellent electrical and thermal conductivity, large surface area and chemical stability. Higher performance, more functions, lower cost and lower toxicity are the development direction of multifunctional inorganic nanomaterials for future energy applications. Although they have great potential, the scientific community still needs to make great efforts to achieve their implementation and success in large-scale applications. The working mechanism of multifunctional inorganic nanomaterials is still fuzzy. Therefore, in-depth characterization using advanced instruments and methods combined with scientific interpretation models should be adapted to gain more insights into the structure–properties relationship of multifunctional inorganic nanomaterials. The functions of devices are closely related to the properties of materials.
Discovering novel materials is hampered by the lack of efficient approaches to the exploration of both a large number of possible elemental compositions and candidate structures.234,235 Artificial intelligence (AI) is currently a hot research method, and is inextricably linked to the future development of multifunctional inorganic materials. In the field of materials, the use of AI to screen different combinations of elements in a fixed structure is a new way to discover novel materials and to simulate material properties.236,237 At present, the exploration of novel materials is still empirical, and the screening of elemental combinations is influenced by human bias. Researchers often focus on the combination of several elements that they find interesting. AI can efficiently screen all possible combinations of elements, which could give a small number of candidate materials and greatly improve the efficiency of finding novel materials.
Finally, multifunctional inorganic nanomaterials intended for energy applications will move towards higher performance and more functions in the future. Core–shell nanomaterials show significant material advantages over individual materials in many energy applications. However, it is still difficult to fabricate high-quality core–shell hollow materials that satisfy all the requirements of energy applications. Compounding materials can significantly improve the performance of materials. The recombination of materials allows the functions of the individual materials to complement each other, resulting in a composite material with multiple functions at the same time. Screening the elements and combination of materials and predicting the properties of materials through AI is an important development direction. Although considerable achievements in multifunctional inorganic nanomaterials have been made in the fields of energy applications which clearly highlight their scope for future development, we still need to constantly strive to create higher performance multifunctional inorganic nanomaterials to meet future demands.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors greatly acknowledge the National Natural Science Foundation of China (Grant No. 51832007).References
- D. Kennedy, Science, 2014, 346, 13 CrossRef CAS PubMed.
- P. D'Odorico, K. F. Davis, L. Rosa, J. A. Carr, D. Chiarelli, J. Dell'Angelo, J. Gephart, G. K. MacDonald, D. A. Seekell, S. Suweis and M. C. Rulli, Rev. Geophys., 2018, 56, 456–531 CrossRef.
- Y. X. Bai, B. Y. Shen, S. L. Zhang, Z. X. Zhu, S. L. Sun, J. Gao, B. H. Li, Y. Wang, R. F. Zhang and F. Wei, Adv. Mater., 2019, 31, 1800680 CrossRef.
- R. Lee, Science, 2011, 333, 569–573 CrossRef CAS PubMed.
- International Energy Agency in World Energy Outlook, International Energy Agency, 2011, pp. 546–547, http://www.worldenergyoutlook.org Search PubMed.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- S. Arepalli, Nanomaterials for Energy: Generation, Harvesting, Transmission and Storage, Elsevier, New York, 2014 Search PubMed.
- Y. Zhang, M. Y. Xie, V. Adamaki, H. Khanbareh and C. R. Bowen, Chem. Soc. Rev., 2017, 46, 7757–7786 RSC.
- https://www.britannica.com/technology/energy-conversion .
- G. Knies, Clean Power from Deserts: The DESERTEC Concept for Energy, Water and Climate Security [White Book], Trans-Mediterranean Renewable Energy Cooperation, Bonn, 2007 Search PubMed.
- F. Bonaccorso, L. Colombo, G. H. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef.
- M. F. Hochella, D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Science, 2019, 363, eaau8299 CrossRef PubMed.
- G. Z. Chen, Inter. Mater. Rev., 2017, 62, 173–202 CrossRef CAS.
- W. H. Azmi, M. Z. Sharif, T. M. Yusof, R. Mamat and A. A. M. Redhwan, Renewable Sustainable Energy Rev., 2017, 69, 415–428 CrossRef CAS.
- J. L. Blackburn, A. J. Ferguson, C. Cho and J. C. Grunlan, Adv. Mater., 2018, 30, 1704386 CrossRef PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. B. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- M. F. Hochella, D. W. Mogk, J. Ranville, I. C. Allen, G. W. Luther, L. C. Marr, B. P. McGrail, M. Murayama, N. P. Qafoku, K. M. Rosso, N. Sahai, P. A. Schroeder, P. Vikesland, P. Westerhoff and Y. Yang, Science, 2019, 363, eaau8299 CrossRef PubMed.
- G. Z. Chen, Int. Mater. Rev., 2017, 62, 173–202 CrossRef CAS.
- Y. Bai, H. Jantunen and J. Juuti, Adv. Mater., 2018, 30, 1707271 CrossRef.
- G. J. Tan, M. Ohta and M. G. Kanatzidis, Philos. Trans. R. Soc., A, 2019, 377, 28 CrossRef.
- T. J. Zhu, Y. T. Liu, C. G. Fu, J. P. Heremans, J. G. Snyder and X. B. Zhao, Adv. Mater., 2017, 29, 1605884 CrossRef PubMed.
- L. D. Hicks and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 12727–12731 CrossRef CAS PubMed.
- L. D. Hicks and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 16631–16634 CrossRef CAS PubMed.
- M. R. Burton, T. J. Liu, J. McGettrick, S. Mehraban, J. Baker, A. Pockett, T. Watson, O. Fenwick and M. J. Carnie, Adv. Mater., 2018, 30, 1801357 CrossRef.
- X. L. Shi, A. Wu, T. L. Feng, K. Zheng, W. D. Liu, Q. Sun, M. Hong, S. T. Pantelides, Z. G. Chen and J. Zou, Adv. Energy Mater., 2019, 9, 1803242 CrossRef.
- Y. B. Luo, S. T. Cai, X. Hua, H. J. Chen, Q. H. Liang, C. F. Du, Y. Zheng, J. H. Shen, J. W. Xu, C. Wolverton, V. P. Dravid, Q. Y. Yan and M. G. Kanatzidis, Adv. Energy Mater., 2019, 9, 1803072 CrossRef.
- R. Nunna, P. F. Qiu, M. J. Yin, H. Y. Chen, R. Hanus, Q. F. Song, T. S. Zhang, M. Y. Chou, M. T. Agne, J. Q. He, G. J. Snyder, X. Shi and L. D. Chen, Energy Environ. Sci., 2017, 10, 1928–1935 RSC.
- J. Zhang, D. Wu, D. S. He, D. Feng, M. J. Yin, X. Y. Qin and J. Q. He, Adv. Mater., 2017, 29, 1703148 CrossRef PubMed.
- Z. Z. Luo, X. M. Zhang, X. Hua, G. J. Tan, T. P. Bailey, J. W. Xu, C. Uher, C. Wolverton, V. P. Dravid, Q. Y. Yan and M. G. Kanatzidis, Adv. Funct. Mater., 2018, 28, 1801617 CrossRef.
- G. J. Tan, C. C. Stoumpos, S. Wang, T. P. Bailey, L. D. Zhao, C. Uher and M. G. Kanatzidis, Adv. Energy Mater., 2017, 7, 1700099 CrossRef.
- A. J. Zhang, B. Zhang, W. Lu, D. D. Xie, H. X. Ou, X. D. Han, J. Y. Dai, X. Lu, G. Han, G. Y. Wang and X. Y. Zhou, Adv. Funct. Mater., 2018, 28, 1705117 CrossRef.
- B. A. MacLeod, N. J. Stanton, I. E. Gould, D. Wesenberg, R. Ihly, Z. R. Owczarczyk, K. E. Hurst, C. S. Fewox, C. N. Folmar, K. H. Hughes, B. L. Zink, J. L. Blackburn and A. J. Ferguson, Energy Environ. Sci., 2017, 10, 2168–2179 RSC.
- L. M. Wang, Z. M. Zhang, L. X. Geng, T. Y. Yuan, Y. C. Liu, J. C. Guo, L. Fang, J. J. Qiu and S. R. Wang, Energy Environ. Sci., 2018, 11, 1307–1317 RSC.
- F. Invernizzi, S. Dulio, M. Patrini, G. Guizzetti and P. Mustarelli, Chem. Soc. Rev., 2016, 45, 5455–5473 RSC.
- F. R. Fan, W. Tang and Z. L. Wang, Adv. Mater., 2016, 28, 4283–4305 CrossRef CAS.
- L. Zhang, S. Bai, C. Su, Y. B. Zheng, Y. Qin, C. Xu and Z. L. Wang, Adv. Funct. Mater., 2015, 25, 5794–5798 CrossRef CAS.
- S. A. Han, T. H. Kim, S. K. Kim, K. H. Lee, H. J. Park, J. H. Lee and S. W. Kim, Adv. Mater., 2018, 30, 1800342 CrossRef PubMed.
- K. Xu, J. Li, X. Lv, J. G. Wu, X. X. Zhang, D. Q. Xiao and J. G. Zhu, Adv. Mater., 2016, 28, 8519–8523 CrossRef CAS.
- J. Wen, B. D. Chen, W. Tang, T. Jiang, L. P. Zhu, L. Xu, J. Chen, J. J. Shao, K. Han, W. Ma and Z. L. Wang, Adv. Energy Mater., 2018, 8, 1801898 CrossRef.
- J. N. Deng, X. Kuang, R. Y. Liu, W. B. Ding, A. C. Wang, Y. C. Lai, K. Dong, Z. Wen, Y. X. Wang, L. L. Wang, H. J. Qi, T. Zhang and Z. L. Wang, Adv. Mater., 2018, 30, 1705918 CrossRef.
- C. S. Wu, A. C. Wang, W. B. Ding, H. Y. Guo and Z. L. Wang, Adv. Energy Mater., 2019, 9, 1802906 CrossRef.
- S. Cheon, H. Kang, H. Kim, Y. Son, J. Y. Lee, H. J. Shin, S. W. Kim and J. H. Cho, Adv. Funct. Mater., 2018, 28, 1703778 CrossRef.
- W. Seung, H. J. Yoon, T. Y. Kim, H. Ryu, J. Kim, J. H. Lee, J. H. Lee, S. Kim, Y. K. Park, Y. J. Park and S. W. Kim, Adv. Energy Mater., 2017, 7, 1600988 CrossRef.
- J. Liu, A. Goswami, K. R. Jiang, F. Khan, S. Kim, R. McGee, Z. Li, Z. Y. Hu, J. Lee and T. Thundat, Nat. Nanotechnol., 2018, 13, 112–116 CrossRef CAS.
- S. J. Peng, G. R. Jin, L. L. Li, K. Li, M. Srinivasan, S. Ramakrishna and J. Chen, Chem. Soc. Rev., 2016, 45, 1225–1241 RSC.
- Z. Wang, Z. J. Shi, T. T. Li, Y. H. Chen and W. Huang, Angew. Chem., Int. Ed., 2017, 56, 1190–1212 CrossRef CAS PubMed.
- B. J. Moon, S. J. Kim, S. Lee, A. Lee, H. Lee, D. S. Lee, T.-W. Kim, S.-K. Lee, S. Bae and S. H. Lee, Adv. Mater., 2019, 31, e1901716 CrossRef PubMed.
- P. Zhang, J. Wu, T. Zhang, Y. F. Wang, D. T. Liu, H. Chen, L. Ji, C. H. Liu, W. Ahmad, Z. D. Chen and S. B. Li, Adv. Mater., 2018, 30, 20 Search PubMed.
- W. Li, A. Elzatahry, D. Aldhayan and D. Y. Zhao, Chem. Soc. Rev., 2018, 47, 8203–8237 RSC.
- X. M. Dong, D. Chen, J. S. Zhou, Y. Z. Zheng and X. Tao, Nanoscale, 2018, 10, 7218–7227 RSC.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Horantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, Science, 2016, 351, 151–155 CrossRef CAS PubMed.
- M. Saliba, T. Matsui, K. Domanski, J. Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J. P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Gratzel, Science, 2016, 354, 206–209 CrossRef CAS.
- N. Aristidou, C. Eames, I. Sanchez-Molina, X. N. Bu, J. Kosco, M. S. Islam and S. A. Haque, Nat. Commun., 2017, 8, 15218 CrossRef PubMed.
- T. A. N. Peiris, A. K. Baranwal, H. Kanda, S. Fukumoto, S. Kanaya, L. Cojocaru, T. Bessho, T. Miyasaka, H. Segawa and S. Ito, Nanoscale, 2017, 9, 5475–5482 RSC.
- X. M. Wang, J. M. Li, W. F. Liu, S. F. Yang, C. F. Zhu and T. Chen, Nanoscale, 2017, 9, 3386–3390 RSC.
- Q. Y. Xi, G. Gao, H. Zhou, Y. X. Zhao, C. Q. Wu, L. D. Wang, P. R. Guo and J. W. Xu, Nanoscale, 2017, 9, 6136–6144 RSC.
- T. Y. Zhang, M. I. Dar, G. Li, F. Xu, N. J. Guo, M. Gratzel and Y. X. Zhao, Sci. Adv., 2017, 3, e1700841 CrossRef PubMed.
- X. Zhang, X. D. Ren, B. Liu, R. Munir, X. J. Zhu, D. Yang, J. B. Li, Y. C. Liu, D. M. Smilgies, R. P. Li, Z. Yang, T. Q. Niu, X. L. Wang, A. Amassian, K. Zhao and S. Z. F. Liu, Energy Environ. Sci., 2017, 10, 2095–2102 RSC.
- S. F. Li, C. Yu, J. Yang, C. T. Zhao, M. D. Zhang, H. W. Huang, Z. B. Liu, W. Guo and J. S. Qiu, Energy Environ. Sci., 2017, 10, 1958–1965 RSC.
- X. G. Zhao, J. H. Yang, Y. H. Fu, D. W. Yang, Q. L. Xu, L. P. Yu, S. H. Wei and L. J. Zhang, J. Am. Chem. Soc., 2017, 139, 2630–2638 CrossRef CAS PubMed.
- X. P. Zheng, B. Chen, J. Dai, Y. J. Fang, Y. Bai, Y. Z. Lin, H. T. Wei, X. C. Zeng and J. S. Huang, Nat. Energy, 2017, 2, 17102 CrossRef CAS.
- A. Bashi, S. Shukla, J. H. Lew, S. Shukla, A. Bruno, D. Gupta, T. Baikie, R. Patidar, Z. Akhter, A. Priyadarshi, N. Mathews and S. G. Mhaisalkar, Nanoscale, 2018, 10, 2341–2350 RSC.
- Q. F. Han, Y. T. Hsieh, L. Meng, J. L. Wu, P. Y. Sun, E. P. Yao, S. Y. Chang, S. H. Bae, T. Kato, V. Bermudez and Y. Yang, Science, 2018, 361, 904–908 CrossRef CAS PubMed.
- Y. C. Hu, R. Patterson, R. Lee-Chin, J. H. Zheng, N. Song, L. Hu, G. Conibeer and S. J. Huang, CrystEngComm, 2018, 20, 3381–3387 RSC.
- J. M. Huang, M. L. Lai, J. Lin and P. D. Yang, Adv. Mater., 2018, 30, 1802856 CrossRef PubMed.
- Z. Li, R. Wang, J. Xue, X. Xing, C. Yu, T. Huang, J. Chu, K.-L. Wang, C. Dong, Z. Wei, Y. Zhao, Z.-K. Wang and Y. Yang, J. Am. Chem. Soc., 2019, 141, 17610–17616 CrossRef CAS PubMed.
- C. W. Chen, H. W. Tsai, Y. C. Wang, T. Y. Su, C. H. Yang, W. S. Lin, Z. H. Lin, J. S. Huang and Y. L. Chueh, J. Mater. Chem. A, 2019, 7, 11452–11459 RSC.
- L. P. Zhu, L. F. Wang, F. Xue, L. B. Chen, J. Q. Fu, X. L. Feng, T. F. Li and Z. L. Wang, Adv. Sci., 2017, 4, 1600185 CrossRef PubMed.
- M. J. Speirs, D. M. Balazs, H. H. Fang, L. H. Lai, L. Protesescu, M. V. Kovalenko and M. A. Loi, J. Mater. Chem. A, 2015, 3, 1450–1457 RSC.
- K. Mahmood, B. S. Swain, A. R. Kirmani and A. Amassian, J. Mater. Chem. A, 2015, 3, 9051–9057 RSC.
- H. Choi, J. H. Song, J. Jang, X. D. Mai, S. Kim and S. Jeong, Nanoscale, 2015, 7, 17473–17481 RSC.
- W. Wang, F. Lv, B. Lei, S. Wan, M. C. Luo and S. J. Guo, Adv. Mater., 2016, 28, 10117–10141 CrossRef CAS PubMed.
- Y. H. He, S. Hwang, D. A. Cullen, M. A. Uddin, L. Langhorst, B. Y. Li, S. Karakalos, A. J. Kropf, E. C. Wegener, J. Sokolowski, M. J. Chen, D. Myers, D. Su, K. L. More, G. F. Wang, S. Litster and G. Wu, Energy Environ. Sci., 2019, 12, 250–260 RSC.
- C. C. Duan, R. Kee, H. Y. Zhu, N. Sullivan, L. Z. Zhu, L. Z. Bian, D. Jennings and R. O'Hayre, Nat. Energy, 2019, 4, 230–240 CrossRef CAS.
- J. L. Shui, M. Wang, F. Du and L. M. Dai, Sci. Adv., 2015, 1, e1400129 CrossRef PubMed.
- M. Sial, M. A. U. Din and X. Wang, Chem. Soc. Rev., 2018, 47, 6175–6200 RSC.
- Y. Mori, T. Ando, T. Matsumoto, T. Yatabe, M. Kikkawa, K. S. Yoon and S. Ogo, Angew. Chem., Int. Ed., 2018, 57, 15792–15796 CrossRef CAS PubMed.
- N. L. Yang, Z. C. Zhang, B. Chen, Y. Huang, J. Z. Chen, Z. C. Lai, Y. Chen, M. Sindoro, A. L. Wang, H. F. Cheng, Z. X. Fan, X. Z. Liu, B. Li, Y. Zong, L. Gu and H. Zhang, Adv. Mater., 2017, 29, 1700769 CrossRef PubMed.
- S. Gentil, N. Lalaoui, A. Dutta, Y. Nedellec, S. Cosnier, W. J. Shaw, V. Artero and A. Le Goff, Angew. Chem., Int. Ed., 2017, 56, 1845–1849 CrossRef CAS PubMed.
- M. Karuppannan, Y. Kim, S. Gok, E. Lee, J. Y. Hwang, J. H. Jang, Y. H. Cho, T. Lim, Y. E. Sung and O. J. Kwon, Energy Environ. Sci., 2019, 12, 2820–2829 RSC.
- B. W. Qin, H. M. Yu, J. Jia, C. Jun, X. Q. Gao, D. W. Yao, X. Y. Sun, W. Song, B. L. Yi and Z. G. Shao, Nanoscale, 2018, 10, 4872–4881 RSC.
- F. Lin, K. Wang, Y. H. Tang, J. P. Lai, M. C. Lou, M. H. Huang and S. J. Guo, Chem. Commun., 2018, 54, 1315–1318 RSC.
- Y. C. Li and T. V. Nguyen, J. Power Sources, 2018, 382, 152–159 CrossRef CAS.
- G. Zhang, Q. H. Ji, K. Zhang, Y. Chen, Z. H. Li, H. J. Liu, J. H. Li and J. H. Qu, Nano Energy, 2019, 59, 10–16 CrossRef CAS.
- X. X. Guo, H. T. Du, F. L. Qu and J. H. Li, J. Mater. Chem. A, 2019, 7, 3531–3543 RSC.
- X. Y. Wu, S. Yin, D. F. Xue, S. Komarneni and T. Sato, Nanoscale, 2015, 7, 17048–17054 RSC.
- M. A. Ghausi, J. F. Xie, Q. H. Li, X. Y. Wang, R. Yang, M. X. Wu, Y. B. Wang and L. M. Dai, Angew. Chem., Int. Ed., 2018, 57, 13135–13139 CrossRef CAS PubMed.
- G. Doubek, R. C. Sekol, J. Y. Li, W. H. Ryu, F. S. Gittleson, S. Nejati, E. Moy, C. Reid, M. Carmo, M. Linardi, P. Bordeenithikasem, E. Kinser, Y. H. Liu, X. Tong, C. O. Osuji, J. Schroers, S. Mukherjee and A. D. Taylor, Adv. Mater., 2016, 28, 1940–1949 CrossRef CAS PubMed.
- C. Tang, H. F. Wang, X. Chen, B. Q. Li, T. Z. Hou, B. S. Zhang, Q. Zhang, M. M. Titirici and F. Wei, Adv. Mater., 2016, 28, 6845–6851 CrossRef CAS PubMed.
- L. P. Zhang, C. Y. Lin, D. T. Zhang, L. L. Gong, Y. H. Zhu, Z. H. Zhao, Q. Xu, H. J. Li and Z. H. Xia, Adv. Mater., 2019, 31, 1805252 CrossRef PubMed.
- J. Li, S. G. Chen, N. Yang, M. M. Deng, S. Ibraheem, J. H. Deng, J. Li, L. Li and Z. D. Wei, Angew. Chem., Int. Ed., 2019, 58, 7035–7039 CrossRef CAS PubMed.
- J. Zhang, G. B. Chen, K. Mullen and X. L. Feng, Adv. Mater., 2018, 30, 1800528 CrossRef PubMed.
- H. T. Du, R. M. Kong, X. X. Guo, F. L. Qu and J. H. Li, Nanoscale, 2018, 10, 21617–21624 RSC.
- M. Sun, D. Davenport, H. J. Liu, J. H. Qu, M. Elimelech and J. H. Li, J. Mater. Chem. A, 2018, 6, 2527–2539 RSC.
- C. Wu, Y. H. Zhang, D. Dong, H. M. Xie and J. H. Li, Nanoscale, 2017, 9, 12432–12440 RSC.
- C. Wu, Y. J. Yang, D. Dong, Y. H. Zhang and J. H. Li, Small, 2017, 13, 1602873 CrossRef PubMed.
- Y. J. Sun, X. Zhang, M. C. Luo, X. Chen, L. Wang, Y. J. Li, M. Q. Li, Y. N. Qin, C. J. Li, N. Y. Xu, G. Lu, P. Gao and S. J. Guo, Adv. Mater., 2018, 30, 1802136 CrossRef PubMed.
- H. Zhu, G. H. Gao, M. L. Du, J. H. Zhou, K. Wang, W. B. Wu, X. Chen, Y. Li, P. M. Ma, W. F. Dong, F. Duan, M. Q. Chen, G. M. Wu, J. D. Wu, H. T. Yang and S. J. Guo, Adv. Mater., 2018, 30, 1707301 CrossRef PubMed.
- D. J. Li, J. Kang, H. J. Lee, D. S. Choi, S. H. Koo, B. Han and S. O. Kim, Adv. Energy Mater., 2018, 8, 1702806 CrossRef.
- L. Z. Bu, N. Zhang, S. J. Guo, X. Zhang, J. Li, J. L. Yao, T. Wu, G. Lu, J. Y. Ma, D. Su and X. Q. Huang, Science, 2016, 354, 1410–1414 CrossRef CAS PubMed.
- X. H. Cao, C. L. Tan, X. Zhang, W. Zhao and H. Zhang, Adv. Mater., 2016, 28, 6167–6196 CrossRef CAS PubMed.
- Y. T. Hu, Y. Wu and J. Wang, Adv. Mater., 2018, 30, 1802569 CrossRef PubMed.
- W. Chen, G. D. Li, A. Pei, Y. Z. Li, L. Liao, H. X. Wang, J. Y. Wan, Z. Liang, G. X. Chen, H. Zhang, J. Y. Wang and Y. Cui, Nat. Energy, 2018, 3, 428–435 CrossRef CAS.
- Y. Gogotsi, Nature, 2014, 509, 568–570 CrossRef CAS PubMed.
- R. Steudel and T. Chivers, Chem. Soc. Rev., 2019, 48, 3279–3319 RSC.
- A. A. Franco, A. Rucci, D. Brandell, C. Frayret, M. Gaberscek, P. Jankowski and P. Johansson, Chem. Rev., 2019, 119, 4569–4627 CrossRef CAS PubMed.
- L.-P. Lv, C. Zhi, Y. Gao, X. Yin, Y. Hu, D. Crespy and Y. Wang, Nanoscale, 2019, 11, 13996–14009 RSC.
- C. Hu, M. Y. Li, J. S. Qiu and Y. P. Sun, Chem. Soc. Rev., 2019, 48, 2315–2337 RSC.
- K. F. Chen and D. F. Xue, Sci. China-Technol. Sci., 2015, 58, 1768–1778 CAS.
- K. F. Chen, S. Yin and D. F. Xue, Nanoscale, 2015, 7, 1161–1166 RSC.
- K. F. Chen, C. T. Sun and D. F. Xue, Phys. Chem. Chem. Phys., 2015, 17, 732–750 RSC.
- K. F. Chen and D. F. Xue, J. Colloid Interface Sci., 2014, 436, 41–46 CrossRef CAS PubMed.
- K. F. Chen and D. F. Xue, J. Colloid Interface Sci., 2014, 430, 265–271 CrossRef CAS.
- K. F. Chen and D. F. Xue, J. Colloid Interface Sci., 2014, 424, 84–89 CrossRef CAS PubMed.
- K. F. Chen, Y. Y. Yang, K. Y. Li, Z. S. Ma, Y. C. Zhou and D. F. Xue, ACS Sustainable Chem. Eng., 2014, 2, 440–444 CrossRef CAS.
- X. T. Liang, K. F. Chen and D. F. Xue, Adv. Energy Mater., 2018, 8, 1703329 CrossRef.
- K. F. Chen and D. F. Xue, J. Colloid Interface Sci., 2014, 416, 172–176 CrossRef CAS PubMed.
- X. Chen, K. F. Chen, H. Wang and D. F. Xue, J. Colloid Interface Sci., 2015, 444, 49–57 CrossRef CAS PubMed.
- X. Chen, K. F. Chen, H. Wang and D. F. Xue, Electrochim. Acta, 2014, 147, 216–224 CrossRef CAS.
- K. F. Chen, D. F. Xue and S. Komarneni, J. Power Sources, 2015, 279, 365–371 CrossRef CAS.
- K. F. Chen, S. Y. Song and D. F. Xue, RSC Adv., 2014, 4, 23338–23343 RSC.
- K. F. Chen and D. F. Xue, Nanoscale, 2016, 8, 17090–17095 RSC.
- K. F. Chen, S. Y. Song, K. Y. Li and D. F. Xue, CrystEngComm, 2013, 15, 10367–10373 RSC.
- K. F. Chen and D. F. Xue, CrystEngComm, 2014, 16, 4610–4618 RSC.
- K. F. Chen and D. F. Xue, ACS Appl. Mater. Interfaces, 2016, 8, 29522–29528 CrossRef CAS.
- S. Sun, T. Zhai, C. Liang, S. V. Savilov and H. Xia, Nano Energy, 2018, 45, 390–397 CrossRef CAS.
- K. Z. Li, S. K. Li, F. Z. Huang, X. Y. Yu, Y. Lu, L. Wang, H. Chen and H. Zhang, Nanoscale, 2018, 10, 2524–2532 RSC.
- Q. C. Zhang, W. W. Xu, J. Sun, Z. H. Pan, J. X. Zhao, X. N. Wang, J. Zhang, P. Man, J. B. Guo, Z. Y. Zhou, B. He, Z. X. Zhang, Q. W. Li, Y. G. Zhang, L. Xu and Y. G. Yao, Nano Lett., 2017, 17, 7552–7560 CrossRef CAS.
- X. Wu, Z. C. Han, X. Zheng, S. Y. Yao, X. Yang and T. Y. Zhai, Nano Energy, 2017, 31, 410–417 CrossRef CAS.
- Y. Liu, N. Q. Fu, G. G. Zhang, M. Xu, W. Lu, L. M. Zhou and H. T. Huang, Adv. Funct. Mater., 2017, 27, 1605307 CrossRef.
- Y. Zhao, L. F. Hu, S. Y. Zhao and L. M. Wu, Adv. Funct. Mater., 2016, 26, 4085–4093 CrossRef CAS.
- J. Lopez, D. G. Mackanic, Y. Cui and Z. N. Bao, Nat. Rev. Mater., 2019, 4, 312–330 CrossRef CAS.
- J. Liu, Z. N. Bao, Y. Cui, E. J. Dufek, J. B. Goodenough, P. Khalifah, Q. Y. Li, B. Y. Liaw, P. Liu, A. Manthiram, Y. S. Meng, V. R. Subramanian, M. F. Toney, V. V. Viswanathan, M. S. Whittingham, J. Xiao, W. Xu, J. H. Yang, X. Q. Yang and J. G. Zhang, Nat. Energy, 2019, 4, 180–186 CrossRef CAS.
- Y. M. Sun, N. A. Liu and Y. Cui, Nat. Energy, 2016, 1, 16071 CrossRef CAS.
- J. Lu, T. P. Wu and K. Amine, Nat. Energy, 2017, 2, 17011 CrossRef CAS.
- G. Assat and J. M. Tarascon, Nat. Energy, 2018, 3, 373–386 CrossRef CAS.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- C. T. Sun and D. F. Xue, CrystEngComm, 2016, 18, 1262–1272 RSC.
- C. T. Sun, Y. J. Zhang, S. Y. Song and D. F. Xue, J. Appl. Crystallogr., 2013, 46, 1128–1135 CrossRef CAS.
- S. C. Zhang, Y. L. Xing, T. Jiang, Z. J. Du, F. Li, L. He and W. B. Liu, J. Power Sources, 2011, 196, 6915–6919 CrossRef CAS.
- K. F. Chen, H. Yang, F. Liang and D. F. Xue, ACS Appl. Mater. Interfaces, 2018, 10, 909–914 CrossRef CAS.
- K. F. Chen and D. F. Xue, CrystEngComm, 2017, 19, 1230–1238 RSC.
- K. H. Kim, W. S. Kim and S. H. Hong, Nanoscale, 2019, 11, 13494–13501 RSC.
- Z. M. Zheng, H. H. Wu, H. X. Chen, Y. Cheng, Q. B. Zhang, Q. S. Xie, L. S. Wang, K. L. Zhang, M. S. Wang, D. L. Peng and X. C. Zeng, Nanoscale, 2018, 10, 22203–22214 RSC.
- L. N. Cong, H. M. Xie and J. H. Li, Adv. Energy Mater., 2017, 7, 1601906 CrossRef.
- S. C. Zhang, Z. J. Du, R. X. Lin, T. Jiang, G. R. Liu, X. M. Wu and D. S. Weng, Adv. Mater., 2010, 22, 5378–5382 CrossRef CAS PubMed.
- M. M. Sun, S. C. Zhang, T. Jiang, L. Zhang and J. H. Yu, Electrochem. Commun., 2008, 10, 1819–1822 CrossRef CAS.
- Y. Y. Yin, L. S. Fan, Y. Zhang, N. N. Liu, N. Q. Zhang and K. N. Sun, Nanoscale, 2019, 11, 7129–7134 RSC.
- R. D. Hu, H. A. Zhao, J. L. Zhang, Q. H. Liang, Y. N. Wang, B. L. Guo, R. Dangol, Y. Zheng, Q. Y. Yan and J. W. Zhu, Nanoscale, 2019, 11, 178–184 RSC.
- W. B. Li, J. F. Huang, L. L. Feng, L. Y. Cao and S. W. He, Nanoscale, 2018, 10, 21671–21680 RSC.
- L. F. Shen, Y. Wang, H. F. Lv, S. Q. Chen, P. A. van Aken, X. J. Wu, J. Maier and Y. Yu, Adv. Mater., 2018, 30, 1804378 CrossRef PubMed.
- K. F. Chen and D. F. Xue, Chin. J. Chem., 2017, 35, 861–866 CrossRef CAS.
- Q. Li, J. N. Guo, J. Zhao, C. C. Wang and F. Yan, Nanoscale, 2019, 11, 647–655 RSC.
- Z. H. Li, Q. He, X. Xu, Y. Zhao, X. W. Liu, C. Zhou, D. Ai, L. X. Xia and L. Q. Mai, Adv. Mater., 2018, 30, 1804089 CrossRef PubMed.
- W. G. Chong, Y. H. Xiao, J. Q. Huang, S. S. Yao, J. Cui, L. Qin, C. Gao and J. K. Kim, Nanoscale, 2018, 10, 21132–21141 RSC.
- D. X. Ji, L. Fan, L. L. Li, S. J. Peng, D. S. Yu, J. N. Song, S. Ramakrishna and S. J. Guo, Adv. Mater., 2019, 31, 1808267 CrossRef PubMed.
- Y. J. Li, L. Cui, P. F. Da, K. W. Qiu, W. J. Qin, W. B. Hu, X. W. Du, K. Davey, T. Ling and S. Z. Qiao, Adv. Mater., 2018, 30, 1804653 CrossRef PubMed.
- Y. T. Xu, B. L. Chen, J. Nie and G. P. Ma, Nanoscale, 2018, 10, 17021–17029 RSC.
- H. Zhang, H. Cui, J. Li, Y. Liu, Y. Yang and M. Wang, Nanoscale, 2019, 11, 21532–21541 RSC.
- S. Wang, W. Gao, X. Y. Hu, Y. Z. Shen and L. Y. Wang, Chem. Commun., 2019, 55, 4137–4149 RSC.
- N. DeForest, A. Shehabi, J. O'Donnell, G. Garcia, J. Greenblatt, E. S. Lee, S. Selkowitz and D. J. Milliron, Build. Environ., 2015, 89, 107–117 CrossRef.
- M. Isaac and D. P. van Vuuren, Energy Policy, 2009, 37, 507–521 CrossRef.
- D. M. Kammen and D. A. Sunter, Science, 2016, 352, 922–928 CrossRef CAS.
- W. Q. Wang, X. L. Wang, X. H. Xia, Z. J. Yao, Y. Zhong and J. P. Tu, Nanoscale, 2018, 10, 8162–8169 RSC.
- C. G. Granqvist, A. Azens, P. Heszler, L. B. Kish and L. Osterlund, Sol. Energy Mater. Sol. Cells, 2007, 91, 355–365 CrossRef CAS.
- R. Baetens, B. P. Jelle and A. Gustavsen, Sol. Energy Mater. Sol. Cells, 2010, 94, 87–105 CrossRef CAS.
- D. T. Gillaspie, R. C. Tenent and A. C. Dillon, J. Mater. Chem., 2010, 20, 9585–9592 RSC.
- G. A. Niklasson and C. G. Granqvist, J. Mater. Chem., 2007, 17, 127–156 RSC.
- K. Bon-Ryul, K. H. Kim and H. J. Ahn, Nanoscale, 2019, 11, 3318–3325 RSC.
- L. Liu, K. Du, Z. B. He, T. Wang, X. L. Zhong, T. Ma, J. M. Yang, Y. C. He, G. B. Dong, S. H. Wang and X. G. Diao, Nano Energy, 2019, 62, 46–54 CrossRef CAS.
- H. Z. Li, L. McRae, C. J. Firby, M. Al-Hussein and A. Y. Elezzabi, Nano Energy, 2018, 47, 130–139 CrossRef CAS.
- S. J. Xie, Y. B. Chen, Z. J. Bi, S. S. Jia, X. X. Guo, X. D. Gao and X. M. Li, Chem. Eng. J., 2019, 370, 1459–1466 CrossRef.
- J. X. Liu, B. Chen, C. Y. Fan, F. Shi, S. Ran, J. Y. Yang, X. Song and S. H. Liu, CrystEngComm, 2018, 20, 1509–1519 RSC.
- Q. Gao, X. M. Wu and L. G. Cai, Sol. Energy Mater. Sol. Cells, 2019, 196, 111–118 CrossRef CAS.
- S. Ran, J. X. Liu, F. Shi, C. Y. Fan, B. Chen, H. M. Zhang, L. Yu and S. H. Liu, Sol. Energy Mater. Sol. Cells, 2018, 174, 342–350 CrossRef CAS.
- Y. C. Xin, X. Cao, S. H. Bao, S. D. Ji, R. Li, Y. Yang, H. J. Zhou and P. Jin, CrystEngComm, 2017, 19, 3931–3938 RSC.
- X. Y. Wu, J. T. Wang, G. K. Zhang, K. Katsumata, K. Yanagisawa, T. Sato and S. Yin, Appl. Catal., B, 2017, 201, 128–136 CrossRef CAS.
- A. Gasparotto, G. Carraro, C. Maccato, C. Sada, J. Balbuena, M. Cruz-Yusta, L. Sanchez, N. Vodisek, U. L. Stangarde and D. Barreca, CrystEngComm, 2018, 20, 1282–1290 RSC.
- Z. Q. Tong, S. K. Liu, X. G. Li, L. Q. Mai, J. P. Zhao and Y. Li, Nanoscale, 2018, 10, 3254–3261 RSC.
- R. Saidur, S. N. Kazi, M. S. Hossain, M. M. Rahman and H. A. Mohammed, Renewable Sustainable Energy Rev., 2011, 15, 310–323 CrossRef CAS.
- A. Kasaeian, S. M. Hosseini, M. Sheikhpour, O. Mahian, W. M. Yan and S. Wongwises, Renewable Sustainable Energy Rev., 2018, 96, 91–99 CrossRef CAS.
- D. P. Kulkarni, D. K. Das and R. S. Vajjha, Appl. Energy, 2009, 86, 2566–2573 CrossRef CAS.
- W. H. Chen, H. E. Mo and T. P. Teng, Energy Build., 2018, 174, 380–387 CrossRef.
- X. Hou, M. J. Wang, L. Fu, Y. P. Chen, N. Jiang, C. T. Lin, Z. W. Wang and J. H. Yu, Nanoscale, 2018, 10, 13004–13010 RSC.
- G. Paul, H. Hirani, T. Kuila and N. C. Murmu, Nanoscale, 2019, 11, 3458–3483 RSC.
- R. Sadri, M. Hosseini, S. N. Kazi, S. Bagheri, A. H. Abdelrazek, G. Ahmadi, N. Zubir, R. Ahmad and N. I. Z. Abidin, J. Colloid Interface Sci., 2018, 509, 140–152 CrossRef CAS PubMed.
- S. N. Du, J. L. Sun and P. Wu, Carbon, 2018, 140, 338–351 CrossRef CAS.
- M. Esquivel-Gaon, N. H. A. Nguyen, M. F. Sgroi, D. Pullini, F. Gili, D. Mangherini, A. I. Pruna, P. Rosicka, A. Sevcu and V. Castagnola, Nanoscale, 2018, 10, 6539–6548 RSC.
- M. Z. Sharif, W. H. Azmi, A. A. M. Redhwan, R. Mamat and G. Najafi, J. Therm. Anal. Calorim., 2019, 135, 1285–1297 CrossRef CAS.
- S. K. Singh and J. Sarkar, Int. Commun. Heat Mass Transfer, 2018, 98, 41–48 CrossRef CAS.
- A. M. Pourrahimi, R. T. Olsson and M. S. Hedenqvist, Adv. Mater., 2018, 30, 1703627 Search PubMed.
- H. Rogalla and P. H. Kes, 100 Years of Superconductivity, CRC, London, 2012 Search PubMed.
- M. M. Ugeda, A. J. Bradley, Y. Zhang, S. Onishi, Y. Chen, W. Ruan, C. Ojeda-Aristizabal, H. Ryu, M. T. Edmonds, H. Z. Tsai, A. Riss, S. K. Mo, D. H. Lee, A. Zettl, Z. Hussain, Z. X. Shen and M. F. Crommie, Nat. Phys., 2016, 12, 92–97 Search PubMed.
- X. X. Xi, Z. F. Wang, W. W. Zhao, J. H. Park, K. T. Law, H. Berger, L. Forro, J. Shan and K. F. Mak, Nat. Phys., 2016, 12, 139–143 Search PubMed.
- X. X. Xi, H. Berger, L. Forro, J. Shan and K. F. Mak, Phys. Rev. Lett., 2016, 117, 106801 CrossRef.
- X. X. Xi, L. Zhao, Z. F. Wang, H. Berger, L. Forro, J. Shan and K. F. Mak, Nat. Nanotechnol., 2015, 10, 765–770 CrossRef CAS PubMed.
- A. W. Tsen, B. Hunt, Y. D. Kim, Z. J. Yuan, S. Jia, R. J. Cava, J. Hone, P. Kim, C. R. Dean and A. N. Pasupathy, Nat. Phys., 2016, 12, 208–212 Search PubMed.
- A. Bhaumik and J. Narayan, Nanoscale, 2019, 11, 9141–9154 RSC.
- A. M. Pourrahimi, T. A. Hoang, D. M. Liu, L. K. H. Pallon, S. Gubanski, R. T. Olsson, U. W. Gedde and M. S. Hedenqvist, Adv. Mater., 2016, 28, 8651–8657 CrossRef CAS PubMed.
- A. M. Pourrahimi, L. K. H. Pallon, D. M. Liu, T. A. Hoang, S. Gubanski, M. S. Hedenqvist, R. T. Olsson and U. W. Gedde, ACS Appl. Mater. Interfaces, 2016, 8, 14824–14835 CrossRef PubMed.
- R. Borgani, L. K. H. Paon, M. S. Hedenqvist, U. W. Gedde and D. B. Haviland, Nano Lett., 2016, 16, 5934–5937 CrossRef CAS.
- L. K. H. Pallon, A. T. Hoang, A. M. Pourrahimi, M. S. Hedenqvist, F. Nilsson, S. Gubanski, U. W. Gedde and R. T. Otsson, J. Mater. Chem. A, 2016, 4, 8590–8601 RSC.
- F. Porrati, S. Barth, R. Sachser, O. V. Dobrovolskiy, A. Seybert, A. S. Frangakis and M. Huth, ACS Nano, 2019, 13, 6287–6296 CrossRef CAS PubMed.
- Y. C. Zou, Z. G. Chen, E. Z. Zhang, F. X. Xiu, S. Matsumura, L. Yang, M. Hong and J. Zou, Nanoscale, 2017, 9, 16591–16595 RSC.
- V. Suresh, J. C. Lin, H. J. Liu, Z. L. Zhang, P. C. Chiang, Y. C. Hsun, Y. C. Chen, J. Y. Lin and Y. H. Chu, Nanoscale, 2016, 8, 18454–18460 RSC.
- H. Paik, D. I. Schuster, L. S. Bishop, G. Kirchmair, G. Catelani, A. P. Sears, B. R. Johnson, M. J. Reagor, L. Frunzio, L. I. Glazman, S. M. Girvin, M. H. Devoret and R. J. Schoelkopf, Phys. Rev. Lett., 2011, 107, 240501 CrossRef PubMed.
- C. M. Natarajan, M. G. Tanner and R. H. Hadfield, Supercond. Sci. Technol., 2012, 25, 063001 CrossRef.
- W. Keijers, X. D. A. Baumans, R. Panghotra, J. Lombardo, V. S. Zharinov, R. B. G. Kramer, A. V. Silhanek and J. Van de Vondel, Nanoscale, 2018, 10, 21475–21482 RSC.
- A. Bhaumik, R. Sachan and J. Narayan, Nanoscale, 2018, 10, 12665–12673 RSC.
- M. Bjergfelt, D. J. Carrad, T. Kanne, M. Aagesen, E. M. Fiordaliso, E. Johnson, B. Shojaei, C. J. Palmstrom, P. Krogstrup, T. S. Jespersen and J. Nygard, Nanotechnology, 2019, 30, 294005 CrossRef CAS PubMed.
- R. M. Lutchyn, E. Bakkers, L. P. Kouwenhoven, P. Krogstrup, C. M. Marcus and Y. Oreg, Nat. Rev. Mater., 2018, 3, 52–68 CrossRef CAS.
- A. Q. Wang, C. T. Li, C. Li, Z. M. Liao, A. Brinkman and D. P. Yu, Phys. Rev. Lett., 2018, 121, 237701 CrossRef CAS PubMed.
- A. Fornieri, A. M. Whiticar, F. Setiawan, E. Portoles, A. C. C. Drachmann, A. Keselman, S. Gronin, C. Thomas, T. Wang, R. Kallaher, G. C. Gardner, E. Berg, M. J. Manfra, A. Stern, C. M. Marcus and F. Nichele, Nature, 2019, 569, 89–92 CrossRef CAS PubMed.
- N. A. Gusken, T. Rieger, P. Zellekens, B. Bennemann, E. Neumann, M. I. Lepsa, T. Schapers and D. Grutzmacher, Nanoscale, 2017, 9, 16735–16741 RSC.
- E. J. H. Lee, X. C. Jiang, M. Houzet, R. Aguado, C. M. Lieber and S. De Franceschi, Nat. Nanotechnol., 2014, 9, 79–84 CrossRef CAS PubMed.
- P. Spathis, S. Biswas, S. Roddaro, L. Sorba, F. Giazotto and F. Beltram, Nanotechnology, 2011, 22, 105201 CrossRef CAS PubMed.
- J. C. Bernard, D. A. Modesto, M. S. Medina, A. Zenatti, E. C. Venancio, E. R. Leite, A. J. C. Lanfredi and M. T. Escote, Mater. Res. Express, 2019, 6, 086001 CrossRef.
- Y. Saito, T. Nojima and Y. Iwasa, Nat. Rev. Mater., 2017, 2, 16094 CrossRef CAS.
- Y. Liu, Y. Huang and X. F. Duan, Nature, 2019, 567, 323–333 CrossRef CAS PubMed.
- K. Tran, G. Moody, F. C. Wu, X. B. Lu, J. Choi, K. Kim, A. Rai, D. A. Sanchez, J. M. Quan, A. Singh, J. Embley, A. Zepeda, M. Campbell, T. Autry, T. Taniguchi, K. Watanabe, N. S. Lu, S. K. Banerjee, K. L. Silverman, S. Kim, E. Tutuc, L. Yang, A. H. MacDonald and X. Q. Li, Nature, 2019, 567, 71–75 CrossRef CAS.
- K. L. Seyler, P. Rivera, H. Y. Yu, N. P. Wilson, E. L. Ray, D. G. Mandrus, J. Q. Yan, W. Yao and X. D. Xu, Nature, 2019, 567, 66–70 CrossRef CAS.
- Z. B. Liu, Z. Y. Fei, C. Xu, Y. X. Jiang, X. L. Ma, H. M. Cheng and W. C. Ren, Nanoscale, 2017, 9, 7501–7507 RSC.
- G. Chen, A. L. Sharpe, P. Gallagher, I. T. Rosen, E. J. Fox, L. Jiang, B. Lyu, H. Li, K. Watanabe, T. Taniguchi, J. Jung, Z. Shi, D. Goldhaber-Gordon, Y. Zhang and F. Wang, Nature, 2019, 572, 215–219 CrossRef CAS.
- C. Xu, S. A. Song, Z. B. Liu, L. Chen, L. B. Wang, D. X. Fan, N. Kang, X. L. Ma, H. M. Cheng and W. C. Ren, ACS Nano, 2017, 11, 5906–5914 CrossRef CAS PubMed.
- W. X. Li, C. Z. Gu and P. A. Warburton, J. Nanosci. Nanotechnol., 2010, 10, 7436–7438 CrossRef CAS.
- W. X. Li, C. Z. Gu, A. J. Cui, J. C. Fenton, Q. Q. Jiang, P. A. Warburton and T. H. H. Shen, J. Mater. Res., 2013, 28, 3063–3078 CrossRef CAS.
- R. Cordoba, A. Ibarra, D. Mailly and J. M. De Teresa, Nano Lett., 2018, 18, 1379–1386 CrossRef CAS.
- Y. Zhou, J. L. He, J. Hu and B. Dang, J. Appl. Polym. Sci., 2016, 133, 42863 Search PubMed.
- S. An, M. Liou, K. Y. Song, H. S. Jo, M. W. Lee, S. S. Al-Deyab, A. L. Yarin and S. S. Yoon, Nanoscale, 2015, 7, 17778–17785 RSC.
- M. Jarvid, A. Johansson, R. Kroon, J. M. Bjuggren, H. Wutzel, V. Englund, S. Gubanski, M. R. Andersson and C. Muller, Adv. Mater., 2015, 27, 897–902 CrossRef CAS PubMed.
- M. M. Adnan, E. G. Tveten, J. Glaum, M. H. G. Ese, S. Hvidsten, W. Glomm and M. A. Einarsrud, Adv. Electron. Mater., 2019, 5, 1800505 CrossRef.
- A. M. Pourrahimi, D. Liu, V. Strom, M. S. Hedenqvist, R. T. Olsson and U. W. Gedde, J. Mater. Chem. A, 2015, 3, 17190–17200 RSC.
- M. Jansen, Angew. Chem., Int. Ed., 2002, 41, 3747–3766 CrossRef.
- C. Collins, M. S. Dyer, M. J. Pitcher, G. F. S. Whitehead, M. Zanella, P. Mandal, J. B. Claridge, G. R. Darling and M. J. Rosseinsky, Nature, 2017, 546, 280–284 CrossRef CAS.
- K. T. Butler, D. W. Davies, H. Cartwright, O. Isayev and A. Walsh, Nature, 2018, 559, 547–555 CrossRef CAS.
- K. A. Severson, P. M. Attia, N. Jin, N. Perkins, B. Jiang, Z. Yang, M. H. Chen, M. Aykol, P. K. Herring, D. Fraggedakis, M. Z. Bazan, S. J. Harris, W. C. Chueh and R. D. Braatz, Nat. Energy, 2019, 4, 383–391 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr07008g |
| This journal is © The Royal Society of Chemistry 2020 |