Open Access Article
Open Access ArticleTriterpenoids
Robert A.
Hill
 * and
Joseph D.
Connolly
* and
Joseph D.
Connolly
School of Chemistry, Glasgow University, Glasgow, G12 8QQ, UK. E-mail: bob.hill@glasgow.ac.uk
First published on 14th February 2020
Abstract
Covering 2015. Previous review: Nat. Prod. Rep., 2018, 35, 1294–1329
This review covers the isolation and structure determination of triterpenoids reported during 2015 including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, serratanes, isomalabaricanes and saponins; 320 references are cited.
1. Introduction
Reviews have been published on the biological properties of triterpenoids including hypoglycaemic,1 antiparasitic2 and immunomodulatory3 activities and neutrophil elastase inhibition.4 Triterpenoid saponins have been highlighted for a range of bioactivities5 and for their neuroprotection.6 Reviews have also appeared covering triterpenoids found in Albizia species,7Alstonia scholaris,8 plants of the Amaranthaceae,9Codonopsis species,10Medicago sativa,11Olea europaea12 and Terminalia species.13 Triterpenoids with a five-membered A-ring have also been covered.142. The squalene group
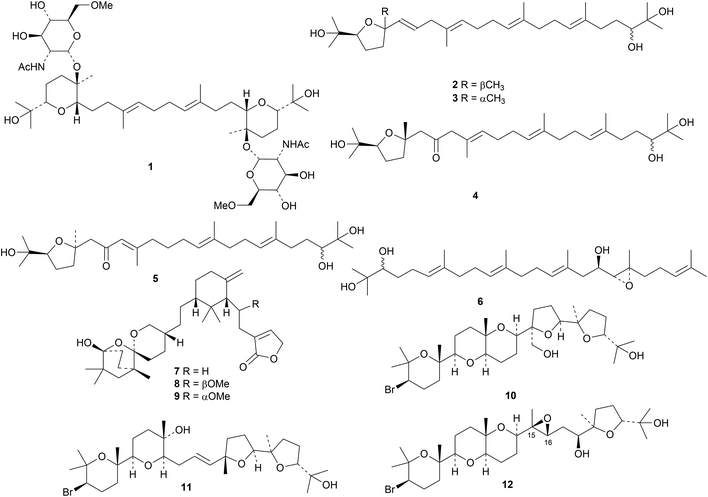
Structure 1 has been proposed for auxarthonoside, a squalene glycoside from the sponge-derived fungus Auxarthron reticulatum.15 Four derivatives, silphasqualols A 2–D 5, have been found in the compass plant Silphium laciniatum.16 A compound from cultures of the basidiomycete Antrodiella albocinnamomea apparently has the non-standard structure 6.17 Saponaceolides Q 7, R 8 and S 9 are further constituents of the European mushroom Tricholoma terreum.18Laurencia viridis is a rich source of squalene derivatives.19 New compounds include 28-hydroxysaiyacenol B 10, saiyacenol C 11 and epoxythyrsiferol A 12 and its 15R,16S-isomer epoxythyrsiferol B. A new pathway for the synthesis of squalene in bacteria has been discovered and the responsible genes identified.203. The lanostane group
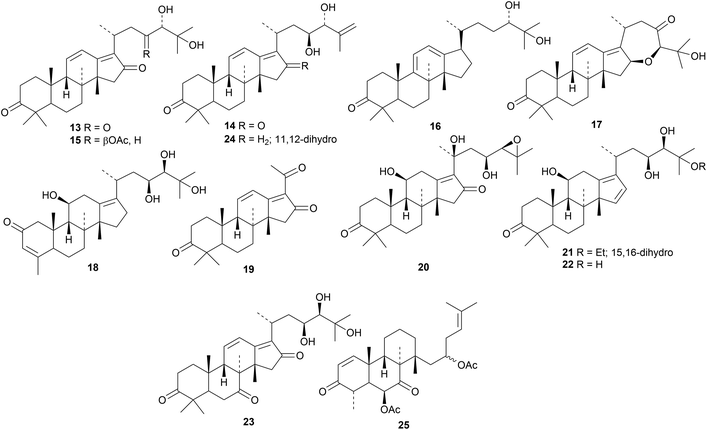
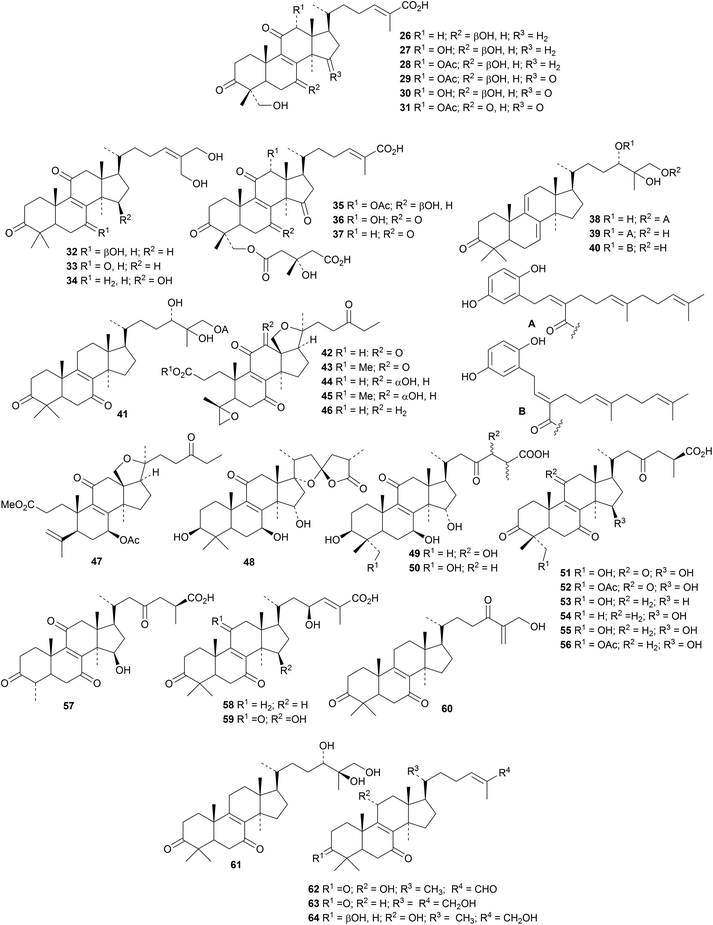
A range of protostane derivatives from the rhizomes of Alisma orientale, that show inhibitory effects on human carboxylesterase 2, includes alismanols A 13–G 19, 20-hydroxyalisol C 20, 25-O-ethylalisol A 21 and compounds 22–24.21 The helvolic acid-related compound 25, from the endophytic fungus Aspergillus fumigatus, has an unusual secofusidane skeleton.22The Tibetan medicinal mushroom Ganoderma leucocontextum is full of lanostanes including ganoleucoins A 26–L 37 and the meroterpenoid esters ganoleucoins M 38–P 41.23 Many of these compounds inhibit HMG-CoA reductase and α-glucosidase. Ganoboninones A 42–F 47 are secolanostanes from the medicinal mushroom Ganoderma boninense that show antiplasmodial activity.24 Constituents of Ganoderma tropicum include25 compounds 48, 49, and 50 while Ganoderma hainanense contains26 ganohainanic acids A 51, its acetate 52, B 53, C 54, D 55, its acetate 56 and E 57. The structure of ganohainanic acid A 51 was confirmed by X-ray crystallographic analysis. Other constituents of this mushroom include hainanic acids A 58 and B 59, the keto alcohols 60 and 61, hainanaldehyde A 62 and compounds 63 and 64.
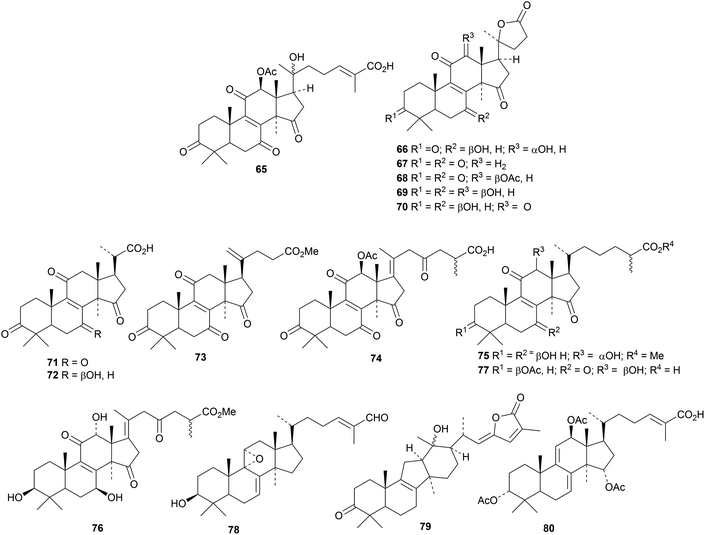
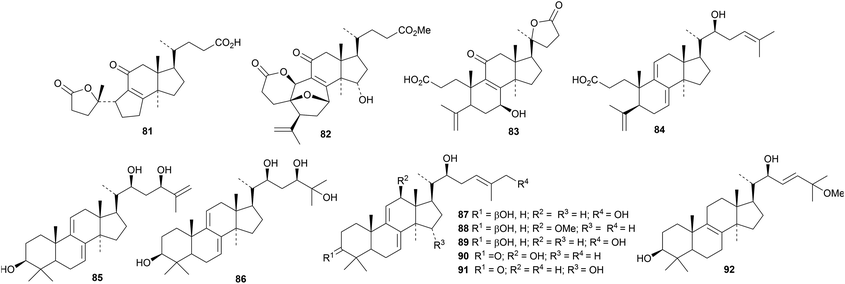
Reviews have appeared highlighting the importance and variety of lanostanes from Ganoderma lucidum.27,28 Newly isolated compounds include ganoderic acid X1 65,29 ganoderlactones A 66–E 70 and ganodernoids A 71–G 77,30 compounds 78 and 79 from the fruiting bodies31 and the triacetate 80.32 Ganocochlearic acid A 81 is an interesting rearranged hexanorlanostane from the fruiting bodies of Ganoderma cochlear where it occurs with cochlate C 82, cochlearic acids A 83 and B 84 and ganodercochlearins D 85–K 92.33 The structures of 83 and 85 were confirmed by X-ray crystallographic analyses.
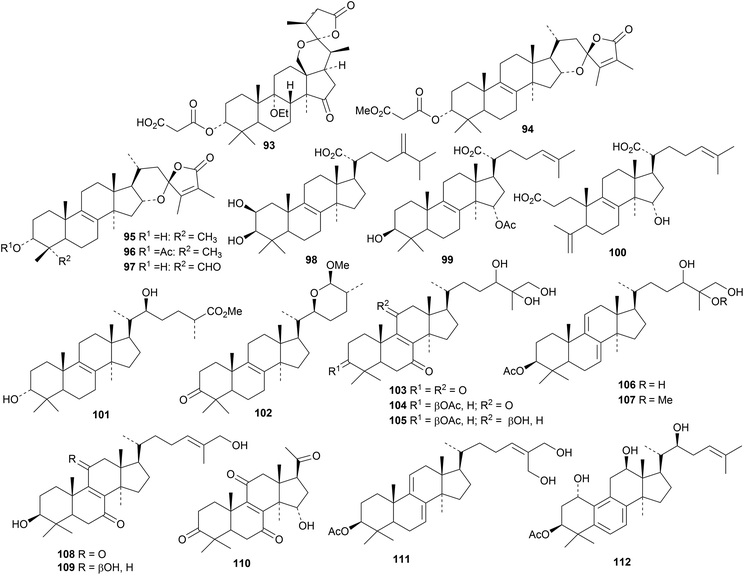
Other fungal products include fomefficinin 93 from Fomes officinalis,34 hexagonins A 94–E 98 from the fruiting bodies of Hexagonia apiaria,35 the 21-oic acid 99, the 3,4-seco 3,21-dioic acid 100 and its 3-methyl ester from the fruiting bodies of Laetiporus sulphureus var. miniatus36 and astrasiate 101 and astrasiaone 102 from the edible mushroom Astraeus asiaticus.37 The metabolites 103–111 of Haddowia longipes ressemble those of Ganoderma species.38 Compound 110 is lucidone H, which is a duplicate name, and 111 is the 3-acetate of ganodermatriol. Diaporthe sp. LG23, an endophytic fungus from Mahonia fortunei, produces the ring B aromatic lanostane 112.39
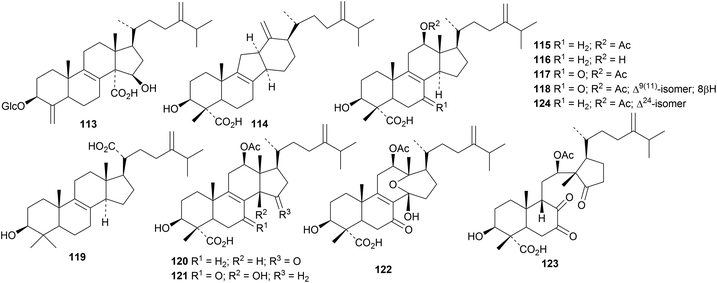
Ascosteroside C 113 is a mitochondrial respiration inhibitor isolated from an Aspergillus species.40 Gloeophyllins A 114–J 123 form an interesting group of normal and rearranged cytotoxic lanostanes from the Tibetan fungus Gloeophyllum abietinum.41 The structures of compounds 114, 115 and 122 were confirmed by X-ray analyses. Gloeophyllin B 115 has also been isolated from Gloeophyllum odoratum, together with the related compound 124.42
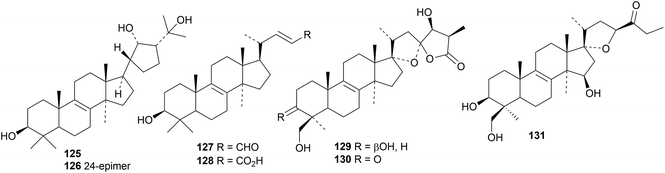
The 21,24-cyclised lanostanes inonotusanes A 125 and B 126 and the trinorderivatives inonotusane C 127 and obliquic acid 128 are constituents of Inonotus obliquus.43 Scillascillol 129 and scillascillone 130 have been isolated from Scilla scilloides.44 The aglycone 131 of bellevalioside D has been found in Eucomis vandermerwei.45
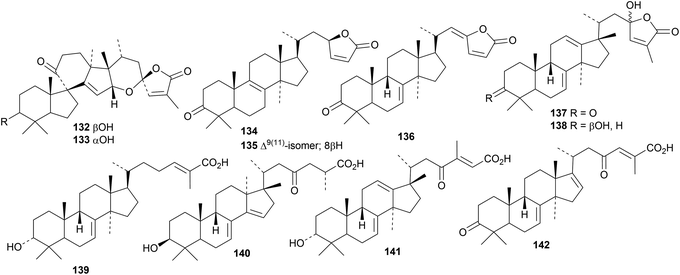
Spirochensilides A 132 and B 133 are rearranged lanostanes from Abies chensiensis.46 The structure of spirochensilide A 132 was confirmed by X-ray analysis. Two groups of compounds, neoabieslactones G 134–K 138 and abiestrines K 139–M 142, have been reported from Abies faxoniana.47 Neoabieslactone I 136 shows interesting topoisomerase II inhibitory activity.
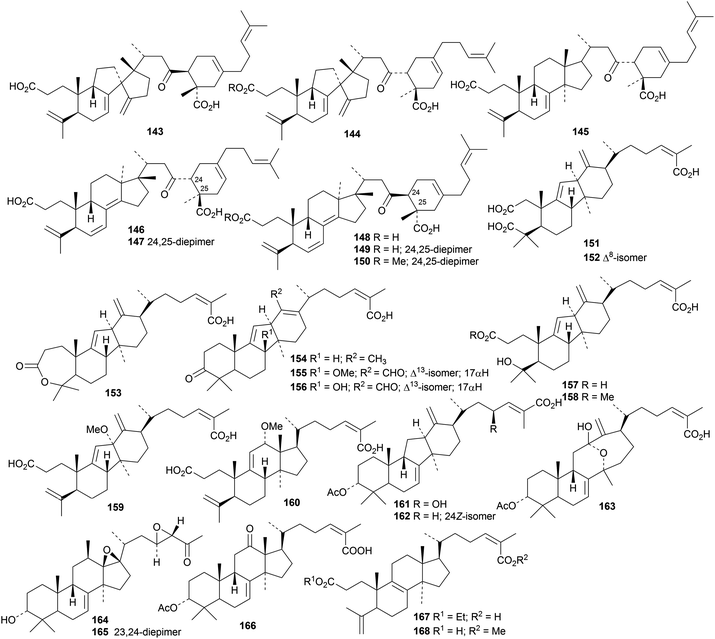
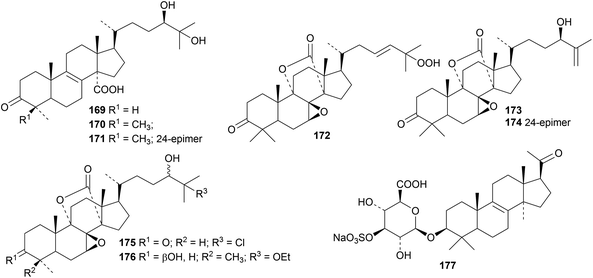
DFT calculations and ROESY data have been used in the stereochemical assignment of abibalsamins C 143–I 150 from the oleoresin of Abies balsamea.48 The abibalsamins are Diels–Alder adducts of lanostane and rearranged lanostane triterpenoids with the monoterpenoid myrcene. Kadsura coccinea is the source of two interesting groups of lanostane derivatives, kadcoccinic acids A 151–J 160 (ref. 49) and kadcoccinones A 161–F 176.50 The structures of 153, 161, 164, 165 and 166 were all confirmed by X-ray crystallographic analyses. Ethyl manwuweizate 167 and methyl manwuweizate 168 are 3,4-secolanostane derivatives from Kadsura heteroclita.51
Compounds 169–176 are minor constituents of a Vietnamese Penares species.52 The saponin 177, with a new hexanorlanostane genin, has been reported from the sponge Clathria gombawuiensis.53 Lanostane saponins with known genins include eryloside W from Dictyonella marsilii,54 scillascilloside B1 from Scilla scilloides44 and saponins from Mussaenda luteola.55
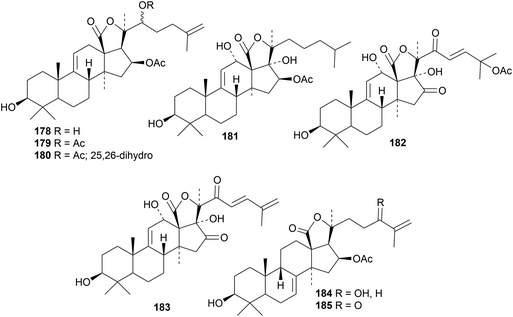
The holostane saponins cladolosides C3, E1, E2, F1, F2, G, H1 and H2, from the sea cucumber Cladolabes schmeltzii, have the new genins 178 (C3), 179 (E2, F2, G, H2) and 180 (E1, F1, H1).56 The saponins lessoniosides A, B and D, with the new genin 181, C and E, with the new genin 182, and F and G, with the new genin 183, have been reported from the viscera of the sea cucumber Holothuria lessoni.57 Among the saponins of the sea cucumber Colochirus robusta, colochirosides B1 and B3 have the new genins 184 and 185 respectively whereas colochirosides B2 and C have known genins.58 The C-22 configuration of the saponins of the sea cucumber Cladolabes schmeltzii has been assigned as R.59 Other holostane saponins with known genins include cercodemasoides A–E from Cercodemas anceps,60 cucumarioside E from Cucumaria japonica61 and moebioside A from Holothuria moebii.62 Reviews covering biological and taxonomic significance of sea cucumber holostane saponins63 and their antitumour, anti-inflammatory and immunostimulatory properties64 have been published.
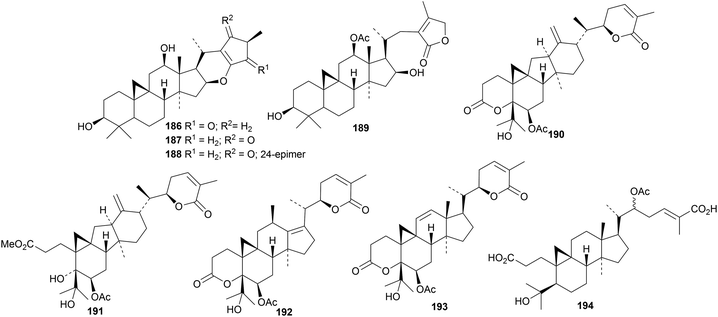
Cimyunnins A 186–D 189 are cycloartane derivatives with interesting side-chains, from the fruit of Cimicifuga yunnanensis.65 A mixture of 187 and 188 was used to confirm the structures by X-ray crystallographic analysis. Cimyunnin A 186 is a potent angiogenesis inhibitor. Ananosins A 190–E 194, cycloartanes from Kadsura ananosma, have some unusual structures.66
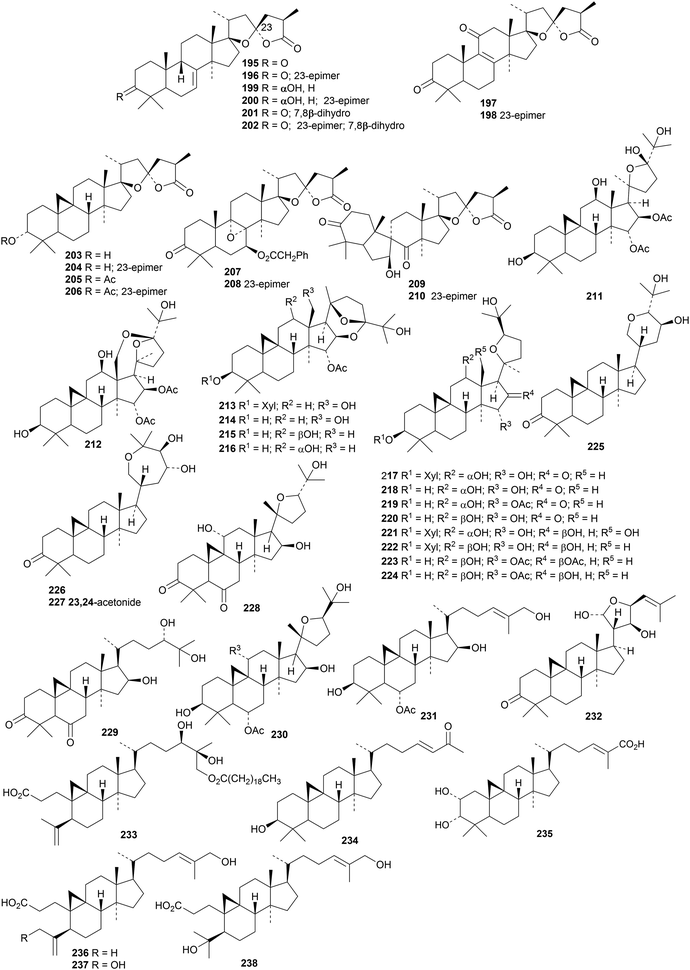
Abies faxoniana is the source of an impressive group of pairs of lanostane and cycloartane derivatives A1/A2195/196–H1/H2209/210, with epimeric spiro-side chains.67 Fourteen new cycloartane derivatives, 211–216 (ref. 68) and 217–224,69 have been isolated from Beesia calthifolia. Other cycloartanes include 225, 226 and 227 from the leaves and twigs of Dysoxylum gotadhora,70 huangqiyegenins V 228 and VI 229 together with the saponins huangqiyenins K and L with the new genins 230 and 231, respectively, from the leaves of Astragalus membranaceus,71 the 21-epimer 232 of the known argenteanone A from the leaves of Lansium domesticum,72 the 3,4-seco derivative 233 from the leaves of Hopea odorata,73 and mangiferenes A 234 and B 235 from Mangifera foetida.74 The structures of 225 and 232 were confirmed by X-ray crystallographic analyses. The 3,4-seco-cycloartanes macrocoussaric acids D 236, E 237 and F 238 have been isolated from Coussarea macrophylla.75
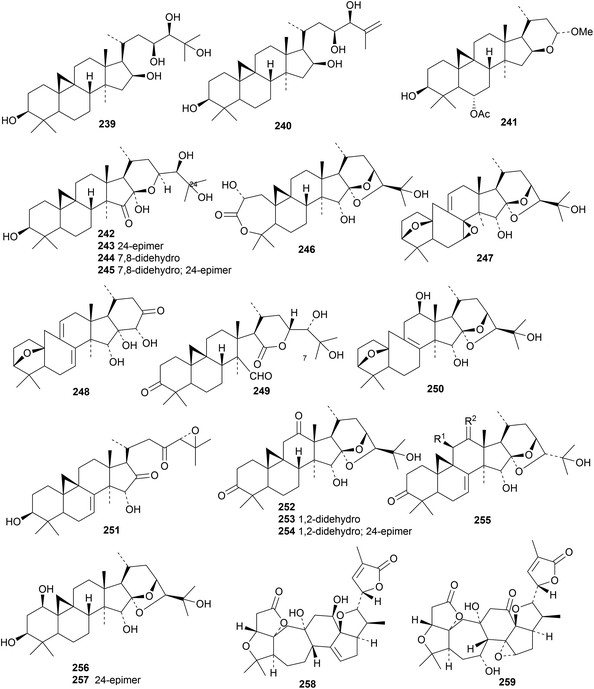
Gambosides A–F are cycloartane glycosides from Astragalus gombo.76 Gambosides A, B and F have the new genins 239, 240 and 241, respectively. Interest in the cycloartane saponins from Cimicifuga simplex continues.77 Glycosides from the aerial parts of Cimicifuga simplex include the new genins 242–245.78 The structure of the 3-O-β-D-xylopyranoside of 244 was confirmed by X-ray crystallographic analysis. Compounds reported from Cimicifuga heracleifolia include cimiheracleins A 246–D 249, 12β-hydroxyacerinol 250, 11-dehydroxy-15α-hydroxycimicidanol 251, cimigenol-3,12-dione 252, the related enones 253 and 254, compound 255 and 1β-hydroxycimigenol 256 and its 24-epimer 257.79 Two new 18-norschiartane derivatives, wuwezidilactones A 258 and B 259, have been reported from Schisandra lancifolia.80 Two reviews of triterpenoids from Schizandra species have been published.81,82 Cycloartane saponins with known genins include amphipaniculosides C and D from Amphilophium paniculatum83 and saponins from Cimicifuga foetida84 and Mussaenda luteola.55
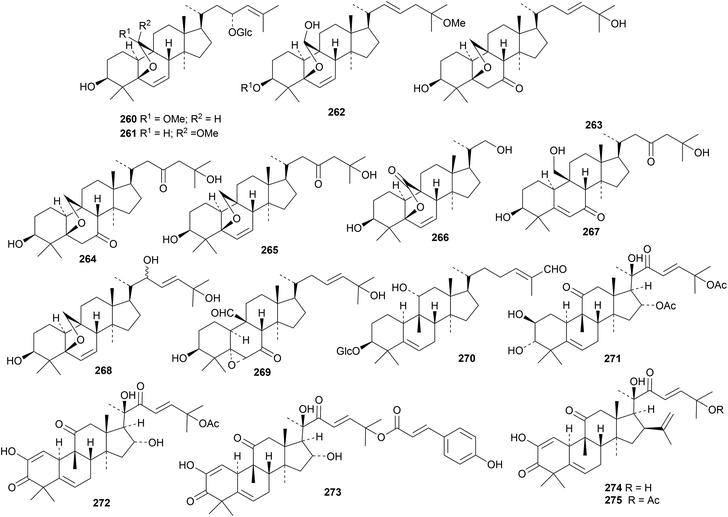
The cucurbitacins have interesting pharmaceutical potential.85,86 Cucurbitacin E has been investigated for a wide range of activities.87 The biosynthesis of the bioactive mogrosides from Siraitia grosvenorii has been studied.88 Three papers describe further cucurbitacin constituents of Momordica charantia, including taikugausins C 260, D 261 and E 262,89 kuguacins II 263–VI 267 (ref. 90) and kaguacin X 268.91 The structure of kuguacin II 263 was confirmed by X-ray crystallographic analysis. Citriodora A 269 has been obtained from Eucalyptus citriodora.92 Hemsleypenside B 270 and the 16,25-diacetate 271 of cucurbitacin F have been isolated from Hemsleya jinfushanensis.93 Hemsleypenside B 270 has a new genin. Other cucurbitacin saponins with the new genins 272 and 273 (ref. 94) and 274 and 275 (ref. 95) have been isolated from the leaves and fruit of Citrullus colocynthis. Genins 274 and 275 have an unusual three carbon unit at C16. Cucurbitacin saponins with known genins include mogroside VA1 from Siraitia grosvenorii,96 kaguaglycoside I91 and taikugausins A and B89 from Momordica charantia and hemsleypensides C, D and E from Hemsleya jinfushanensis.93
4. The dammarane group
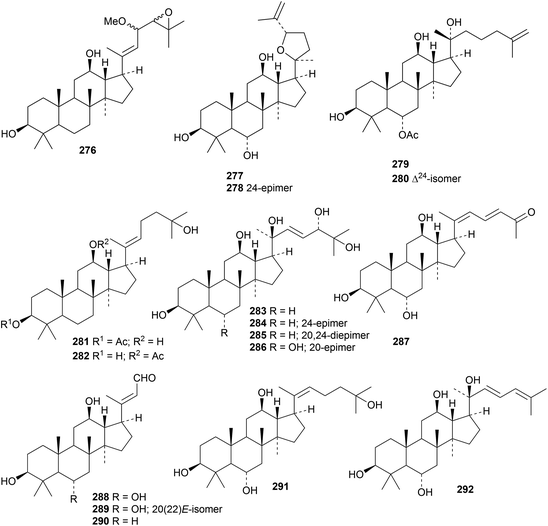
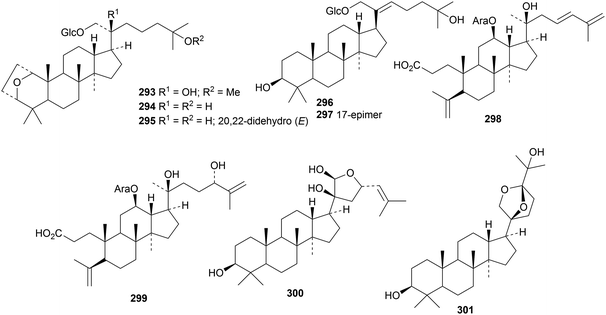
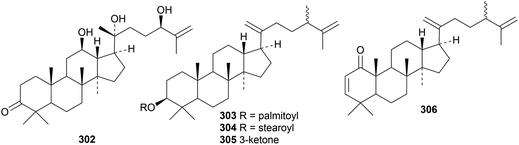
The pharmaceutical use of dammarane triterpenoids has been reviewed.97 Reviews have also appeared on the saponins from Panax ginseng98 and Panax notoginseng.99,100 Four new ginsenosides, with melanogenesis inhibitory activity, have been isolated from Panax ginseng but only one of them, 23-O-methylginsenoside Rg11, has a new genin 276.101 Six new dammaranes 277–282 have been obtained from the acidic hydrolysate of the stems and leaves of Panax ginseng.102 The steamed roots of Panax notoginseng yielded notoginsenosides SP1–SP4 with the new genins 283–286 (ref. 103) and notoginsenosides ST7–ST10, 13 and 14 with the genins 287–292.104Other dammarane derivatives include gypensapogenins H 293–L 297 from the hydrolysate of the total saponins of Gynostemma pentaphyllun,105 cyclocariosides I 298 (duplicate name) and J 299 from the leaves of Cyclocarya paliurus,106 glylongiposides I and II, with the new genins 300 and 301, from Gynostemma longipes,107 compound 302 from the green walnut husks of Juglans mandshurica108 and compounds 303–306 from Cissus quadrangularis.109
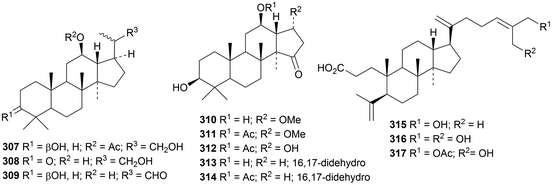
Two groups of nordammarannes, compounds 307, 308 and 309 from Sanguisorba officinalis110 and hupehenols A 310–E 314 from Viburnum hupehensis,111 have been reported. Macrocoussaric acids A 315, B 316 and C 317 are 3,4-secodammaranes from the Ecuadorian plant Coussarea macrophylla.75
Epoxynotoginsenoside A is a saponin from Panax notoginseng that is identical to the previously isolated quinquefoloside Lb.112 Dammarane saponins with known genins include actinostemmosides I and J, from Actinostemma lobatum,113 cyclocarioside K from Cyclocarya paliurus,106 20S-ginsenoside RF2 from Panax ginseng,114 notoginsenosides SP5–SP18 (ref. 103) and notoginsenosides ST6, ST11 and ST12 from Panax notoginseng104 and saponins from Gynostemma pentaphyllum115 and Panax ginseng.116–118 The current knowledge of the key enzymes involved in the biosynthesis of saponins from Panax notoginseng has been surveyed.119
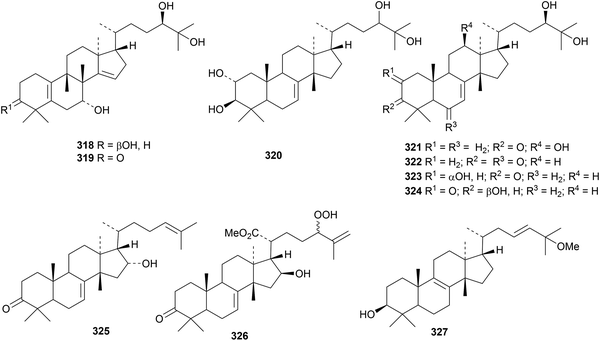
Ricinidols A 318 and B 319 have a new rearranged euphane skeleton.120 They occur in Ricinodendron heudelotii together with the euphane derivatives ricinodols C 320–G 324. The structure of 16-epikulinone 325, from Melia azedarach, was established by X-ray crystallographic analysis.121 Other euphanes include the antibacterial toosendanin A 326 from Melia toosendan122 and 25-methoxy-8,23-euphadien-3β-ol 327 from Euphorbia pekinensis.123
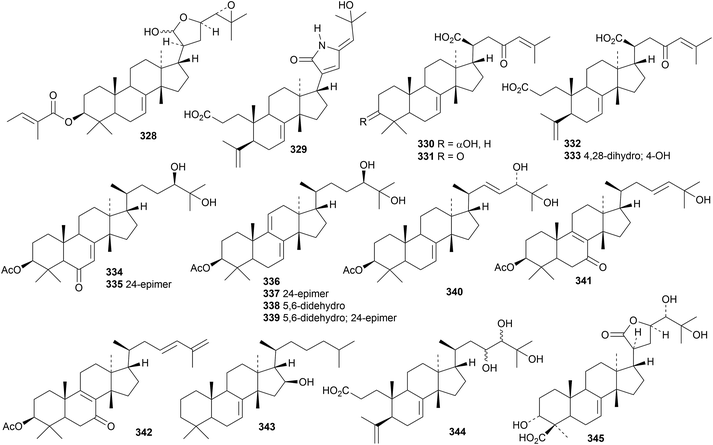
New tirucallane derivatives include 3β-O-tigloylmelianol 328 from Guarea kunthiana,124 congoensins A 329 and B 330 from the bark of Entandrophragma congoënse,125 compounds 331, 332 and 333 from Anopyxis klaineana,126 ficutirucins A 334–I 342 from the fruit of Ficus carica,127 24,25-dihydrolimocinol 343 from Melia azadirachta128 and secotiaminic acid 344 from Entandrophragma congoense.129 Paramigniosides A–E are tirucallane saponins from Paramignya scandens, all with the same genin 345.130
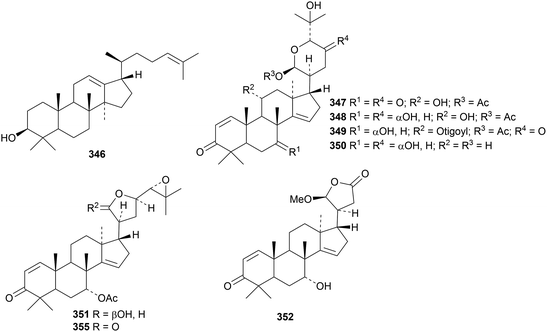
Polygonifoliol 346, from the latex of the seaside sandmat Euphorbia polygonifolia, is an apotirucallane with an 18(17→14)-abeo rearrangement.131 Piscidinols H 347–L 351 are apotirucallane derivatives from the leaves of Walsura trifoliata that show moderate insecticidal activities.132 The apotirucallol derivative 352 has been isolated from the seeds of Xylocarpus granatum.133
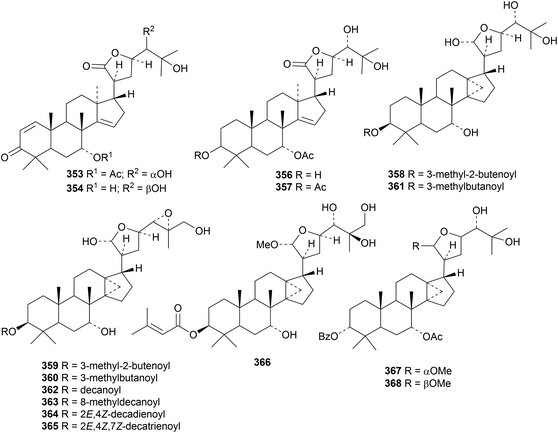
The apotirucallane and glabretal derivatives prototiamins A 353–G 359 are constituents of the bark of Entandrophragma congoënse.129 The structure of prototiamin C 355 was confirmed by X-ray analysis. Other glabretal derivatives include dictabretals A 360–D 363 from the root bark of Dictamnus dasycarpus,134 pancastatins A 364 and B 365 from the immature fruit of Poncirus trifoliata,135 compound 366 from Atalantia buxifolia136 and dysomollins A 367 and B 368 from Dysoxylum mollissimum var. glaberrimum.137
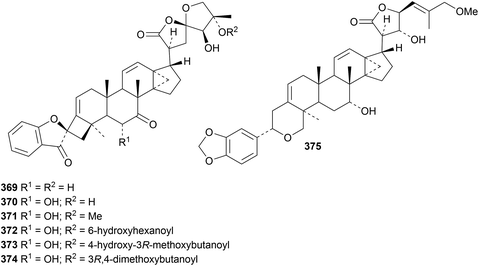
Phainanoids A 369–F 374, from Phyllanthus hainanensis, have an interesting new carbon skeleton.138 The structures of A and B were confirmed by X-ray crystallographic analyses. Phainanoids A 369–F 374 exhibited potent immunosuppressive activities. Songbodichapetalin 375 is a new constituent of Phyllanthus songboiensis with cytotoxic activity.139
4.1. Tetranortriterpenoids
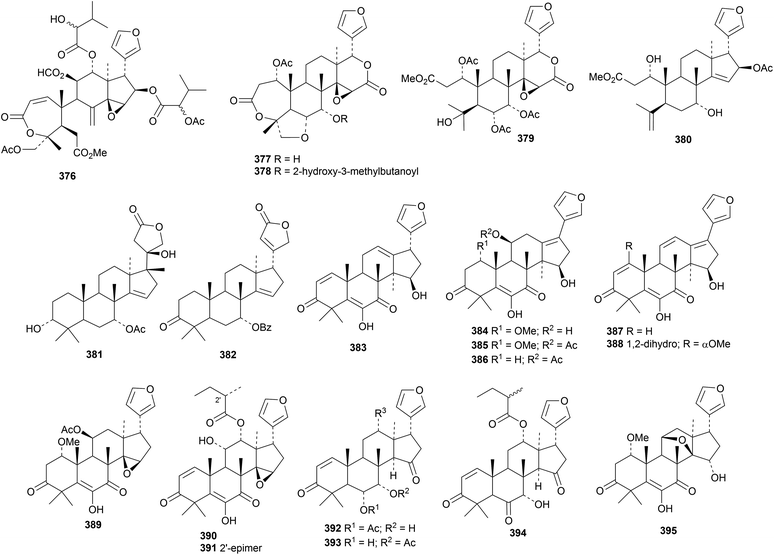
Many new limonoids have been reported this year. Dysomollides A 376–G 382 are constituents of Dysoxylum mollissimum var. glaberrimum.137Dysoxylum mollissimum is also the source of dysoxylumosins A 383–M 395.140The leaves of Trichilia americana are rich in cedrelone derivatives, including americanolides A 396–D 399 and compounds 400–405.141 The structure of americanolide A 396 was confirmed by X-ray crystallographic analysis.
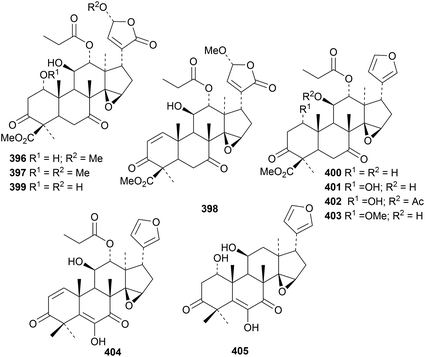
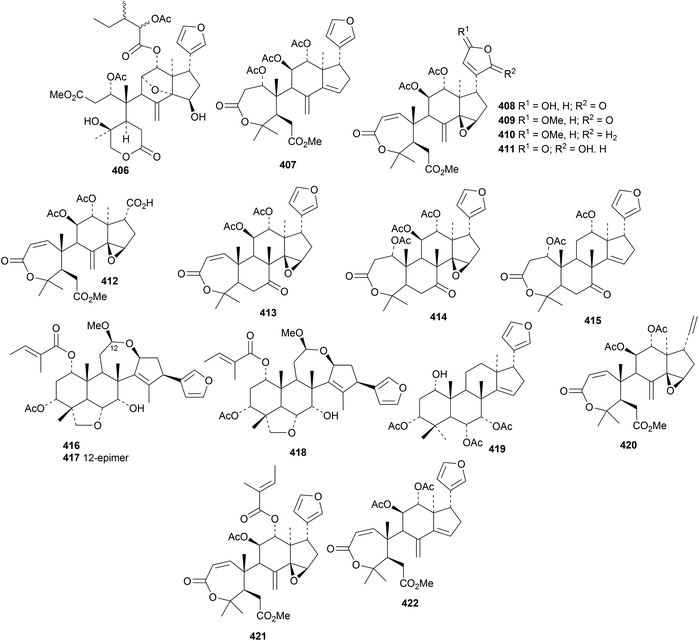
Munronins A 406–N 419 (ref. 142) and O 420, P 421 and Q 422 (ref. 143) are constituents of Munronia henryi. Unfortunately, the names munronins A–G have been used before and munronin L 415 is the known 23-O-methylvolkensin. The structure of munronin H 412 was confirmed by X-ray crystallographic analysis. Many of the munronins show strong anti-tobacco mosaic virus activity.
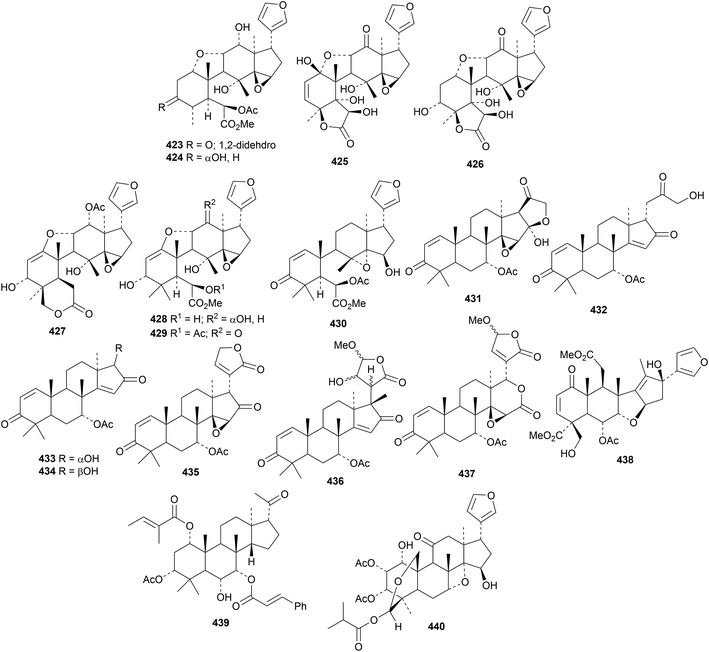
Several 29-nor and related limonoids, toonaciliatones A 423–H 430, have been found in the stem bark of Toona ciliata var. yunnanensis.144 Toonaciliatones A–F are names that have already been used. New compounds from Azadirachta species include azadiraindins A 431–D 434,145 E 435, F 436 and G 437 (ref. 146) and nimbolicidin 438 and nimbocin 439 (ref. 147) from Azadirachta indica and mehaneemin 440 from Azadirachta excelsa.148
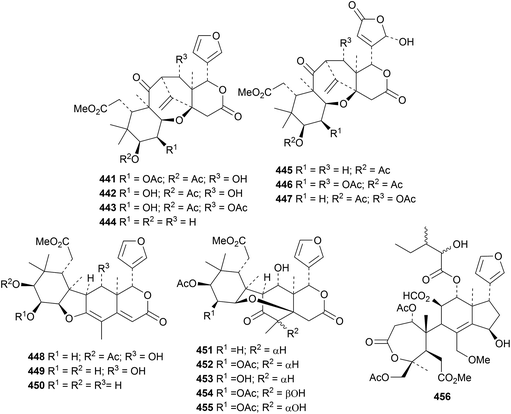
Ciparasins A 441–P 456 constitute an impressive array of rearranged limonoids from Cipadessa cinerascens.149 Ciparasins A 441 and P 456 show strong anti-HIV activities.
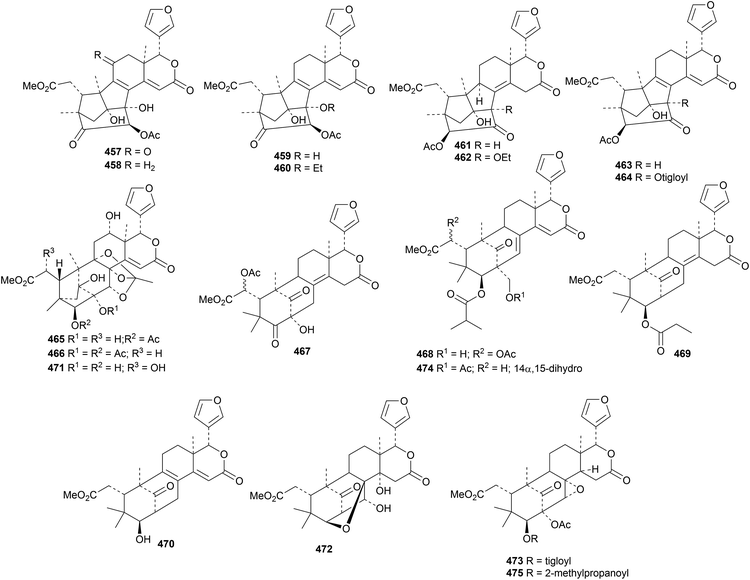
The Thai mangrove Xylocarpus moluccensis is the source of thaixylomolins G 457–N 464, the xyloccensin U derivatives 465 and 466 and the mexicanolide derivatives 467and 468,150 while the seeds of Indian Xylocarpus moluccensis yielded proceranolide propanoate 469 and deacetylangustidienolide 470.151 2,3-Dideacetylxyloccensin S 471 and 30-deacetylxyloccensin W 472 have been isolated from the seeds of Xylocarpus granatum.133 The seeds of Swietenia macrophylla152 and Swietenia humilis153 are the respective sources of swietemacrophin 473 and humilinolides G 474 and H 475. Swietemacrophin 473 is the same as the previously isolated 2-acetylruageanin B and 2-acetoxyswietemahonolide. The structure of humilinolide G 474 was confirmed by X-ray crystallographic analysis.
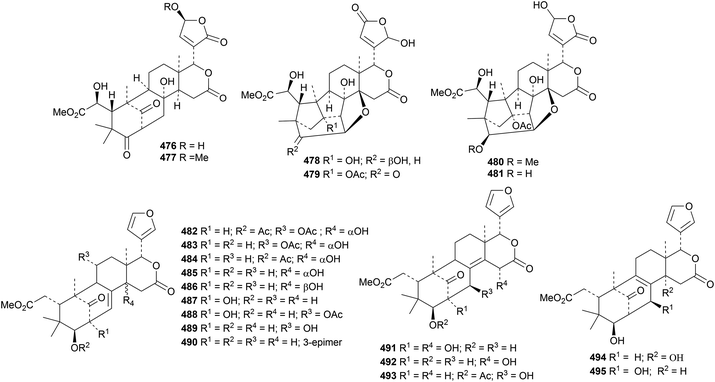
Khaysenelides A 476–F 481 are modified furan derivatives from the stem bark of Khaya senegalensis.154 The structures of khaysenelides A 476 and C 478 were confirmed by X-ray crystallographic analyses. Khaysenelide F 481 shows neuroprotective activity. An impressive group of mexicanolide derivatives, khasenegasins A 482–N 495, has been reported from the seeds of Khaya senegalensis.155
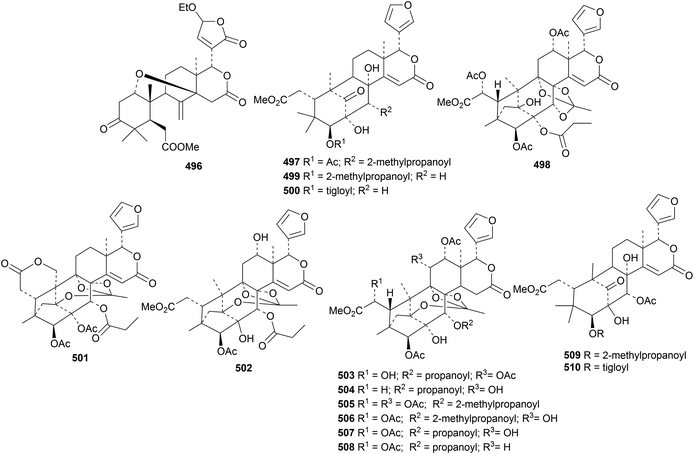
New compounds from Carapa guianensis (andiroba) include andirolides W 496, X 497 and Y 498 from the flower oil156 and carapanolides T 499–X 503 (ref. 157) and M 504–S 510 (ref. 158) from the seeds. The structures of khasenegasin A 482 and carapanolide N 505 were confirmed by X-ray crystallographic analyses. Carapanolide V 501 is the same as andirolide F, isolated in 2011.
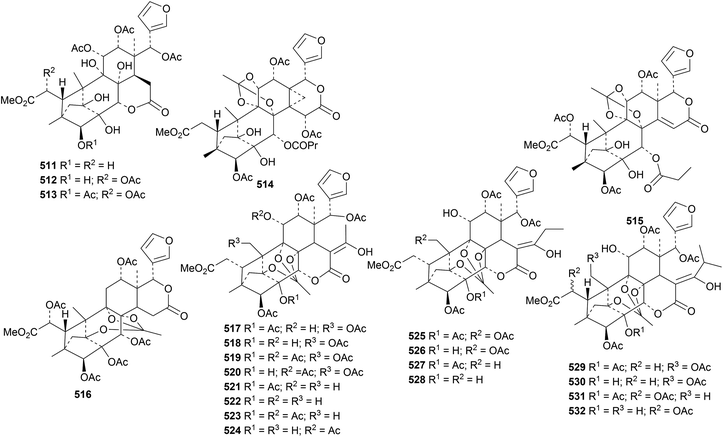
Tabulalins K 511, L 512 and 513 (ref. 159) and velutabularins K 514, L 515 and M 516 (ref. 160) are new phragmalin derivatives from Chukrasia tabularis var. velutina. The structure of velutabularin K 514 was confirmed by X-ray crystallographic analysis. Further phragmalin derivatives, chukvelutilides I 517–X 532, have been isolated from Chukrasia tabularis.161 This paper adds to the confusion in the literature by using duplicate names (chukvelutilides I–O). In addition, chukvelutilides U and V are the same as the previously published chukvelutilides I and L.
4.2. Quassinoids
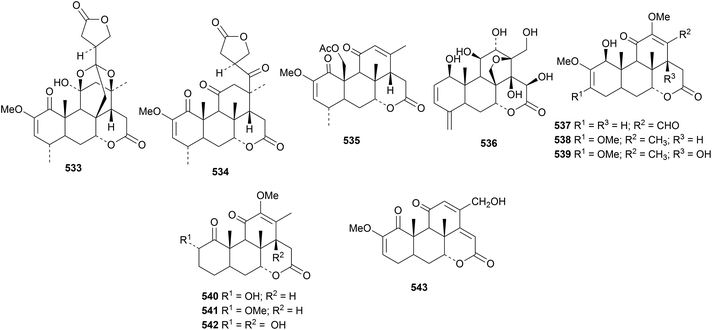
Perforalactones A 533, B 534 and C 535 are interesting new quassinoids from Harrisonia perforata.162 Perforalactones A 533 and B 534 have been shown to have insecticidal activity but no cytotoxic activity. Brucea javanica yielded the new derivative bruceene A 536.163 The structures of perforalactone A 533 and bruceene A 536 were confirmed by X-ray analyses. Several new quassinoids, picrajavanicins A 537–G 543, have been reported from Picrasma javanica, collected in Myanmar.1645. The lupane group
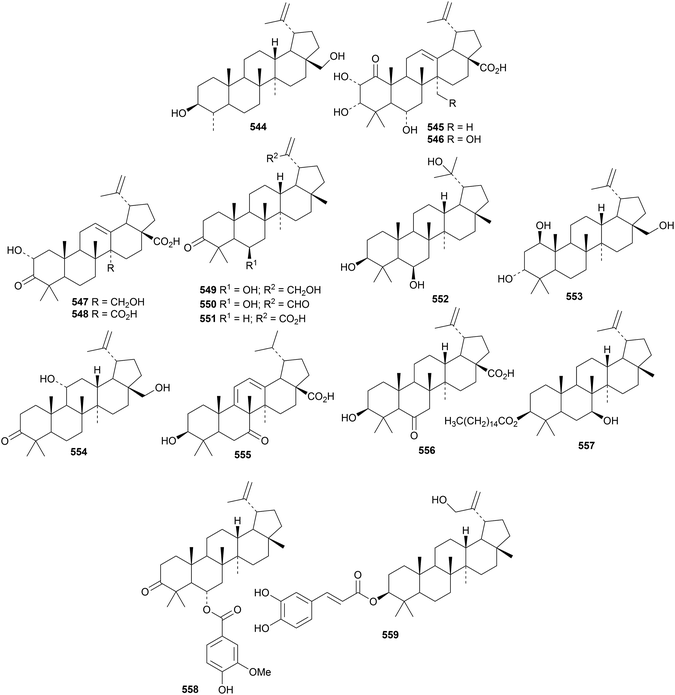
24-Norbetulin 544 has been identified in Dracaena cinnabari.165 The highly oxygenated lupane derivatives 545 and 546 are constituents of cashew nuts (Anacardium occidentale).166 The related compounds 547 and 548 have been found in Egyptian apple peel (Malus domestica).167,168Cassine xylocarpa is the source of four new lupane derivatives 549–552 and a further two, 553 and 554, are from Maytenus cuzcoina.169 Other new lupane derivatives include the antibacterial 3β-hydroxy-9(11),12-lupadien-28-oic acid 555 from Sonneratia alba170 and 3β-hydroxy-7-oxo-20(29)-lupen-28-oic acid 556 from Manilkara zapota.171 Novel lupane esters include the 3-hexadecanoyl ester 557 of 20(29)-lupene-3β,7β-diol from Scurrula parasitica parasitic on Nerium indicum,172 the 4-hydroxy-3-methoxybenzoyl ester 558 from Paullinia pinnata,173 the cafeoyl ester 559 from Celastrus stylosus174 and the 2-benzoyl ester of alphitolic acid from Rubus innominatus175 Hancokinoside, from Allophylus africanus, is the 2-O-β-D-glucopyranoside of the known hancokinol although the diagram in the reference is inaccurate.176 Other lupane saponins with known genins include royleanumioside from Teucrium royleanum177 and a saponin from Cyperus rotundus.1786. The oleanane group
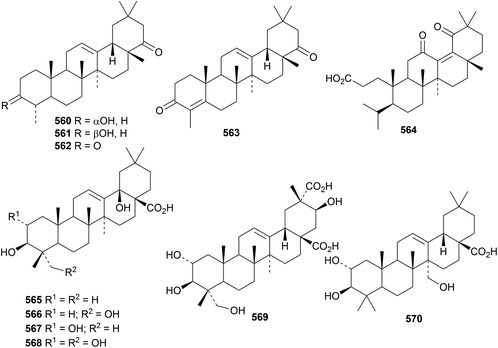
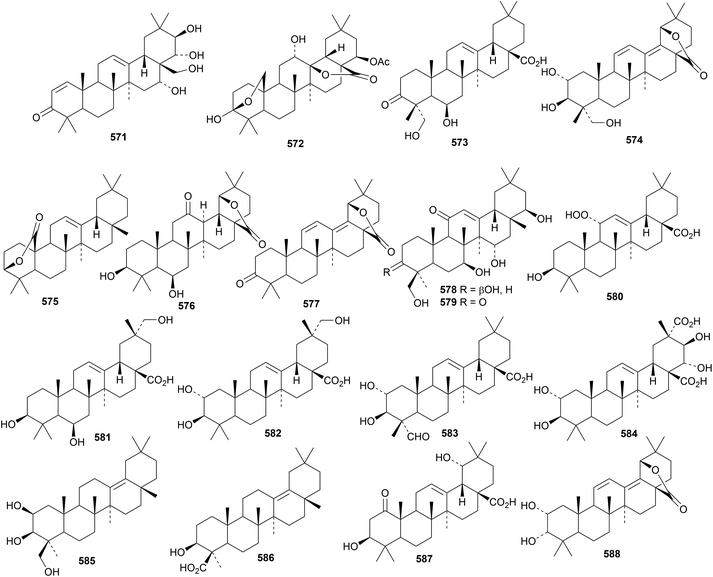
Three 24-noroleananes, 560, 561 and 562, have been isolated from biogas slurry.179 The structure of 24-nor-12-oleanene-3,22-dione 562 was confirmed by X-ray crystallographic analysis. The related dione 563 has also been found in biogas slurry.180 The 3,4-seco-oleanane derivative 564, from Hypericum ascyron, contains an unusual enedione.181 Four oleananes 565–568 with unusual hydroxylation at C18 have been isolated from hawthorn berries (Crataegus pinnatifida).182 Oleananes 566, 567 and 568 exhibited strong antiproliferative activity. Brachyanthera acid A 569, from Stautonia brachyanthera, is 2α,3β,21β,23-tetrahydroxy-12-oleanene-28,29-dioic acid183 and pashia acid 570, from Pyrus pashia, is 2α,3β,27-trihydroxy-12-oleanen-28-oic acid.184Other new oleanane derivatives include cyrillin A 571 from Cyrilla racemiflora,185 lancamarolide 572 from Lantana camara,186 silphanolic acid C 573 from Silphium laciniatum,16 termichebulolide 574 from Terminalia chebula,187 ulubelenolide 575 from Tanacetum chiliophyllum var. monocephalum,188 the 28,19-olides 576 and 577 from Styrax tonkinensis,189 the pentahydroxyoleanenone 578 and the corresponding 3-ketone 579 from Gueldenstaedtia verna,190 the 11-hydroperoxide 580 from Holarrhena curtisii,191 3β,6β,29-trihydroxy-12-oleanen-28-oic acid 581 from Spermacoce latifolia,192 2α,3β,29-trihydroxy-12-oleanen-28-oic acid 582 and related compounds 583 and 584 from Akebia trifoliata,193 the 12(18)-enes 585 and 586 from Schnabelia oligophylla194 and 1-oxosiaresinolic acid 587 and 2α,3α-dihydroxy-11,13(18)-oleanadien-28,19β-olide 588 from Rubus innominatus.175
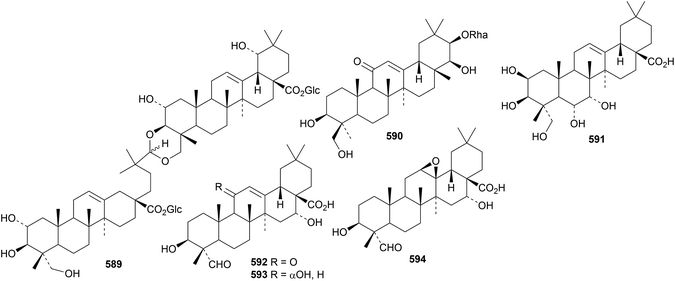
Termichebuloside A 589, a dimeric oleanane diglucosyl ester from Terminalia chebula, is the 4,4′-diepimer of the known ivorenoside A.187 The rhamnoside 590, from Gueldenstaedtia verna has a new genin.190 Pachystelanoside B is a saponin from Pachystela msolo with the new genin 7α-hydroxyprotobassic acid 591.195 Silenorubicosides E–I are saponins from Silene rubicunda with the new genins 592 (E, F and G), 593 (H) and 594 (I).196
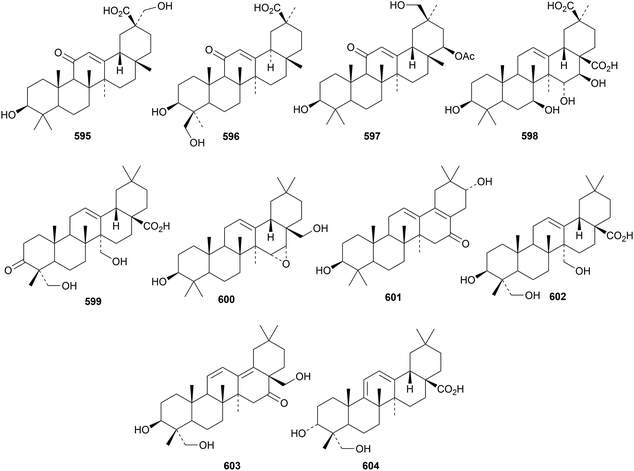
Licoricesaponins P2 and Q2 are saponins from Glycyrrhiza inflata with the new genins 595 and 596, respectively.197 Genin 596 has an unusual 18αH. Other oleanane saponins with new genins include 22-acetyluralsaponin C from Glycyrrhiza uralensis with genin 597,198 atrioleanoside from Atriplex lasiantha with genin 598,199 indicacin from Fagonia indica with genin 599 (ref. 200) and schisusaponins G and H from Schima superba with genins 600 and 601, respectively,201 and saponins from Anemone amurensis with genin 602,202Bupleurum chinense with genin 603 (ref. 203) and Ilex cornuta with genin 604.204
New oleanane saponins with known genins that have been assigned trivial names are listed in Table 1.
| Trivial name | Plant species | Reference |
|---|---|---|
| Abyssaponins A, B | Erythrina abyssinica | 205 |
| Aesculiosides S1, S2 | Aesculus sylvatica | 206 |
| Akeqintoside E | Akebia quinata | 207 |
| Amphipaniculosides A | Amphilophium paniculatum | 83 |
| Aquaticasaponins A, B | Gleditsia aquatica | 208 |
| Ardinuloside | Ardisia insularis | 209 |
| Bigelovii C | Salicornia bigelovii | 210 |
| Bigelovii D | Salicornia bigelovii | 211 |
| Boromoenosides A–D | Albizia boromoensis | 212 |
| Brachyantheraosides A1–A5, B6, B9 | Stauntonia brachanthera | 183 |
| Catunarosides I, J | Catunaregam spinosa | 213 |
| Centellasaponin H | Centella asiatica | 214 |
| Clematograveolenoside A | Clematis graveolens | 215 |
| Comastomasaponin I | Comastoma pedunculatum | 216 |
| Coriacea saponin A | Holboellia coriacea | 217 |
| Crotalariosides C, (E/Z)-D, (E/Z)-E, (E/Z)-F | Polygala crotalarioides | 218 |
| Cyrillin B | Cyrilla racemiflora | 185 |
| Enterolacaciamine | Enterolobium cyclocarpum | 219 |
| Floraassamsaponins I–VIII | Camellia sinensis var. assamica | 220 |
| Ginsenoside Ro sulfate | Silphium laciniatum | 16 |
| Glomerulosides A–H | Glochidion glomerulatum | 221 |
| Glomerulosides I, II | Glochidion glomerulatum | 222 |
| Hemslosides Ma4, Ma5 | Hemsleya chinensis | 223 |
| Hippophosides E, F | Hippophae rhamnoides | 224 |
| Lebbeckosides A, B | Albizia lebbeck | 225 |
| Leptocarposides B, C, D | Ludwigia leptocarpa | 226 |
| Lobatoside O | Actinostemma lobatum | 113 |
| Lonicerosides K, L, M | Weigela subsessilis | 227 |
| Macedonoside E | Glycyrrhiza uralensis | 198 |
| Meliomosides A–G | Meliosma henryi | 228 |
| Oleiferasaponins C1, C2, C3 | Camellia oleifera | 229 |
| Oleiferosides I–M | Camellia oleifera | 230 |
| Oleiferosides N, O | Camellia oleifera | 231 |
| Oleiferosides P–T | Camellia oleifera | 232 |
| Pachystelanoside A | Pachystela msolo | 195 |
| Perennisosides XIII–XIX | Bellis perennis | 233 |
| Poliusaposides A, B, C | Teucrium polium | 234 |
| Rotundinoside A | Ilex rotunda | 235 |
| Serjanioside D | Serjania marginata | 236 |
| Schefflerasides A, B | Schefflera sessiliflora | 237 |
| Schekwangsiensides Ia, Ib, IIa, IIb, III–VI, VIIa, VIIb, VIII | Schefflera kwangsiensis | 238 |
| Schisusaponins A–F | Schima superba | 201 |
| Securiosides C, D, E | Securidaca inappendiculata | 239 |
| Stauntosides G, H | Stauntonia obovatifoliola | 240 |
| Vulgaside I | Prunella vulgaris | 241 |
| Zanhasaponins D–H | Zanha golungensis | 242 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2.
| Plant species | Reference |
|---|---|
| Acanthophyllum laxiusculum | 243 |
| Anemone amurensis | 202 |
| Anemone taipaiensis | 244 |
| Bupleurum chinense | 203 |
| Calendula officinalis | 245 |
| Callicarpa kwangtungensis | 246 |
| Eclipta prostrata | 247 |
| Entada phaseoloides | 248 |
| Eryngium kotschyi | 249 |
| Gueldenstaedtia verna | 190 |
| Lecythis pisonis | 250 |
| Phryna ortegioides | 251 |
| Phyllanthus myrtifolius | 252 |
| Pittosporum tobira | 253 |
| Polygala crotalarioides | 254 |
| Planchonella obovata | 255 |
| Sanguisorba officinalis | 256 |
| Saponaria officinalis | 257 |
| Trifolium argutum | 258 |
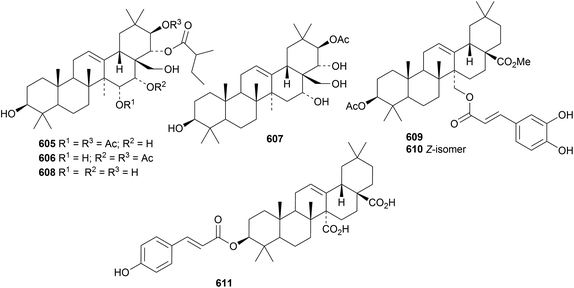
New oleanane esters from Barringtonia racemosa include racemosol B 605 and isoracemosol B 606 (ref. 259) and racemosols C 607 and D 608.260 Other new oleanane esters include the formyl ester of β-amyrin from Dichrocephala benthamii,261 the caffeoyl esters 609 and 610 from Waltheria indica262 and the coumaroyl ester 611 from Astilbe rivularis.263
There have been many reports of the biological activities of oleanane triterpenoids and their saponins including the antitumour activity264 and stem cell differentiation activity265 of oleanolic acid and the antitumour activity of ardipusilloside,266 boswellic acids,267 β-escin268 and raddeanin A.269 The biological properties of glycyrrhizic acid270 and other triterpenoids271 from Glycyrrhiza glabra have been reviewed.
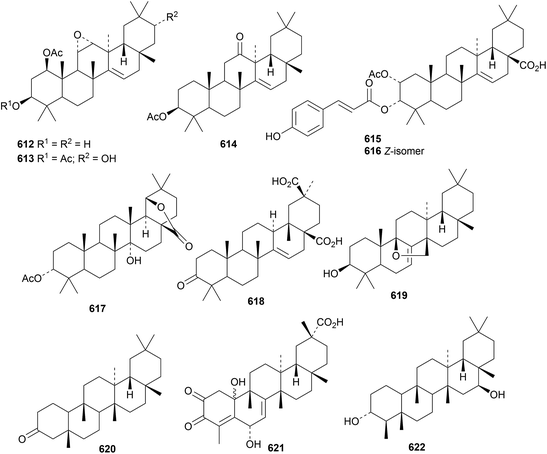
Genicunolides A 612 and B 613 are taraxerane 11,12-epoxides from Euphorbia geniculata.272 The octacosanoyl ester of taraxerol has been found in Clerodendrum philippinum var. simplex.273 3β-Acetoxy-14-taraxeren-12-one 614 is a constituent of Manilkara zapota.171 The coumaroyl esters maesculentins A 615 and B 616 have been isolated from Manihot esculenta.274 The taraxastane 617 has been reported from Neoboutonia macrocalyx with unusual stereochemistry at C13 and C18.275 The 27(13→18)-abeotaraxerane derivative 618, from Pittosporum illicioides, is related to isoaleuritolic acid.276 Two taraxerane saponins with known genins have been reported from Euphorbia dracunculoides.277 9β,26-Epoxy-7-multiforen-3β-ol 619 has been isolated from Trichosanthes baviensis.278 Hainanenone A 620, from Drypetes hainanensis, is 23-nor-3-friedelanone279 and the 24-norfriedelane 621 has been found in Celastrus stylosus.174Drypetes congestiflora is the source of 3α,16β-friedelanediol 622.280
7. The ursane group
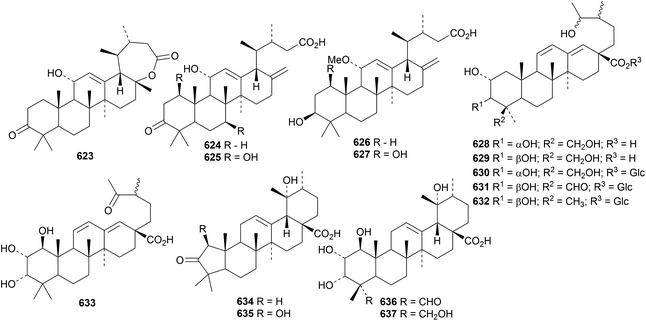
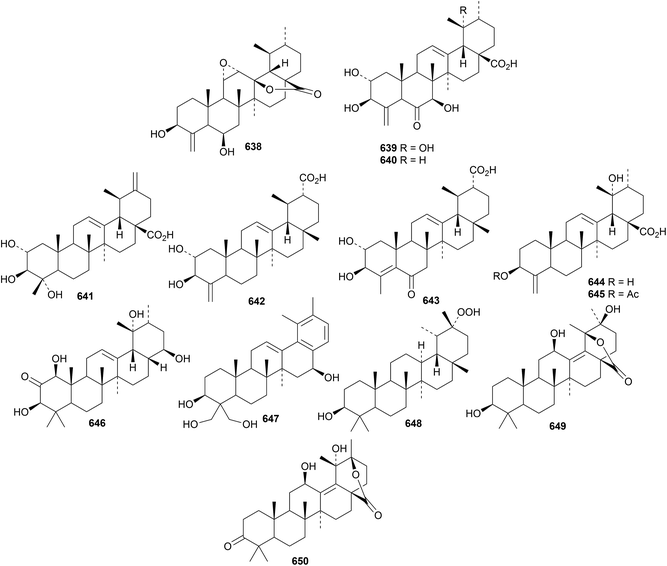
The antitumour properties of ursolic acid have been well studied.281–283 Urmiensolide B 623 and urmiensic acid 624, from Salvia urmiensis, are 17,22-secoursane derivatives that showed significant antiproliferative activity.284 Further 17,22-secoursanes include the apoptosis-inducing 625 from Salvia urmiensis285 and 626 and 627 from Salvia syriaca.286Rubus lambertianus is the source of the 18,19-secoursanes 628 and 629 together with the glucopyranosyl esters 630, 631 and 632.287 The related 18,19-secoursane 633 has been isolated from Rubus innominatus together with the ring-A contracted ursanes rubuminatus A 634 and B 635 and the ursanes 636 and 637.175Hookerinoid C 638 from Pterocephalus hookeri288 and gelsenorursanes A 639–E 643 from Gelsemium elegans289 are 24-norursanes. Further 24-norursanes 644 and 645 have been obtained from Mostuea hirsuta.290 The 28-norursane 646 is a constituent of Agrimonia pilosa291 and the 28-norursane 647, with an aromatic ring E, is present in Gardenia jasminoides var. radicans.292 Ixeritriterpenol 648 has been reported from Ixeris chinensis with unusual stereochemistry at C13 and C19.293 The structure of 3β,12β,20β-trihydroxy-13(18)-ursen-28,19β-olide 649, from Ilex latifolia, was confirmed by X-ray crystallographic analysis.294 The related lactone 650 also occurs in Ilex latifolia.
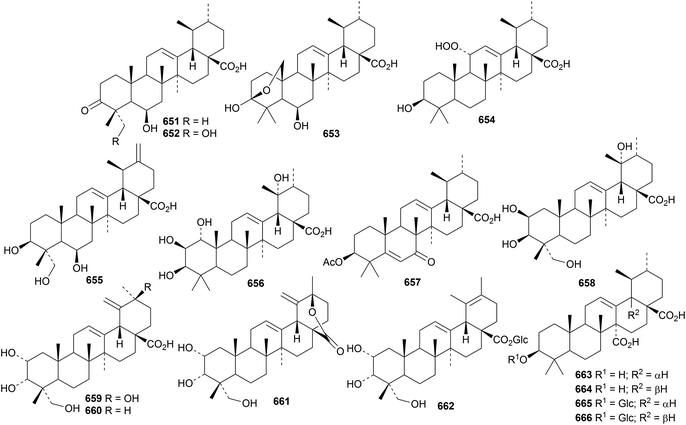
Further simple ursane derivatives include silphanolic acids A 651, B 652 and D 653 from Silphium laciniatum,16 the 11-hydroperoxide 654 from Holarrhena curtisii,191 3β,6β,23-trihydroxy-12,20(30)-ursadien-28-oic acid 655 from Spermacoce latifolia,192 1α,2β,3β,19α-tetrahydroxy-12-ursen-28-oic acid 656 from Vitellaria paradoxa,295 the enone 657 from Codium dwarkense,296 2β,3β,19α,23-tetrahydroxy-12-ursen-28-oic acid 658 and its glycosyl ester from Nauclea officinalis,297 the related compounds 659, 660 and 661 and the glucosyl ester 662 from Oenothera maritima298 and the 27,28-dioic acids 663 and 664 and their 3-glucosides 665 and 666 from Crossopteryx febrifuga.299
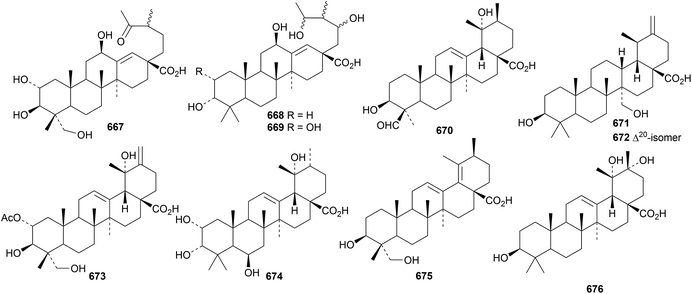
New ursane saponins with new genins include bodiniosides E, F and G with the genins 667, 668 and 669, respectively, from Elsholtzia bodinieri,300 ilexpublesnin S with genin 670, with an unusual 20β configuration, from Ilex pubescens,301 pittangretosides C1 and L with the genins 671 and 672, respectively, from Pittosporum angustifolium302 and vulgaside II with genin 673 from Prunella vulgaris241 and saponins from Callicarpa kwangtungensis with genin 674,246Ilex cornuta with genin 675 (ref. 303) and Ilex kudingcha with genin 676.304
Named ursane saponins with known genins include amphipaniculoside B from Amphilophium paniculatum,83 catunarosides K and L from Catunaregam spinosa,213 centellasaponins F and G from Centella asiatica,214 comastomasaponins J and K from Comastoma pedunculatum216 and rotundinosides B, C and D from Ilex rotunda.235 Unnamed saponins with known genins have been isolated from Cassia siamea,305Clematoclethra scandens ssp. actinidioides,306Firmiana simplex,307Ilex cornuta,204,303,308Ilex kudingcha,304Ilex latifolia294 and Sanguisorba officinalis.256
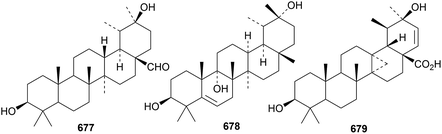
Fagonicin 677, from Fagonia indica, is 3β,20β-dihydroxytaraxastan-28-al with an unusual stereochemistry at C13 (ref. 200) and genicunolide C 678, from Euphorbia geniculata, is 5-taraxastene-3β,9α,20α-triol.272 Ilexpublenin T, from Ilex pubescens is a taraxastane saponin with a known genin301 and a saponin from Clematis uncinata also has a known taraxastane saponin.309 The 13,27-cyclo ursane 679 has been identified in Ochradenus arabicus.310
8. The hopane group
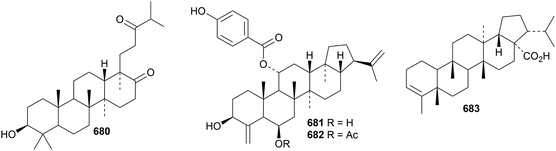
The 17,21-secohopane derivative, scabanol 680, has been isolated from Gentiana scabra.311 Exotheols A 681 and 682, from Exothea paniculata, are 24-norhopane esters.312 3-Filicen-28-oic acid 683 is a constituent of the Chinese fern Lepidogrammitis drymoglossoides.3139. Miscellaneous compounds
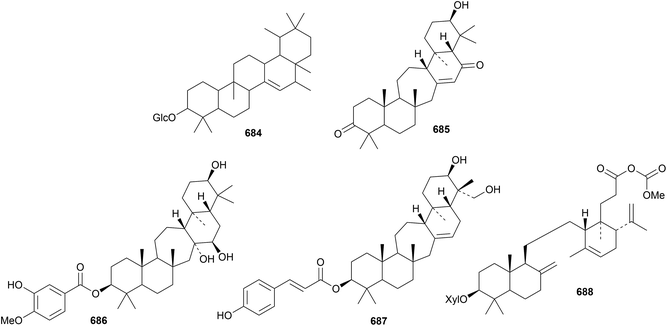
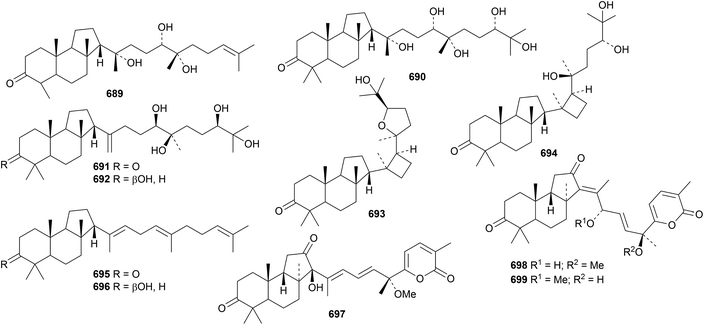
The unlikely structure 684 has been proposed for allotaraxerolide from Allophylus africanus.176 21β-Hydroxy-14-serratene-3,16-dione 685 has been isolated from Lycopodiella cernua together with the serratane esters 686 and 687.314 Lansioside D 688, from Lansium domesticum, is a derivative of the 21,22-seco-onocerane lansiolic acid.315 Lansioside D 688 showed potent activity against Gram-positive bacteria.Six malabaricane derivatives 689–694 have been obtained from Ailanthus malabarica, including two containing cyclobutane rings.316 Further malabaricanes 695 and 696 have been found in Bursera microphylla.317 The isomalabaricane derivatives stellettins N 697, O 698 and P 699 have been identified in extracts of the marine sponge Stelletta tenuis.318 All three stelletins showed significant cytotoxic activities. Stelletin N is a duplicate name.
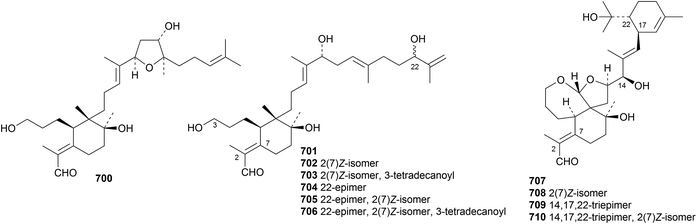
Iris tectorum is the source of several iridal triterpenoids including iritectols C 700–F 703 and the 22-epimers 704, 705 and 706 (ref. 319) and polycycloiridals A 707–D 710.320
10. Conflicts of interest
There are no conflicts of interest.11. References
- K. Zhu, Z. Wu, C. Jiang, L. Cao, J. Zhang and Z. Yin, Zhongguo Yaoke Daxue Xuebao, 2015, 46, 764–770 CAS.
- M. Markowski and W. Janiszowska, Kosmos, 2015, 64, 129–136 CAS.
- K. R. Sharma, A. Adhikari, A. Jabeen, N. Dastagir, S. K. Kalauni, I. M. Choudhary and Y. R. Pokharel, Biochem. Pharmacol., 2015, 4, 1000182 Search PubMed.
- D. Guillaume, T. N. T. Huynh, D. Clement, K. P. P. Nguyen and A. Belaaouaj, Nat. Prod. Commun., 2015, 10, 167–170 CrossRef PubMed.
- V. R. Netala, S. B. Ghosh, P. Bobbu, D. Anitha and V. Tartte, Int. J. Pharm. Pharm. Sci., 2015, 7, 24–28 CAS.
- A. Sun, X. Xu, J. Lin, X. Cui and R. Xu, Phytother. Res., 2015, 29, 187–200 CrossRef CAS PubMed.
- A. N. Singab, D. Bahgat, E. Al-Sayed and O. Eldahshan, Med. Aromat. Plants, 2015, 4, 1/1–1/7 CAS.
- A. Arora and Y. Rai, Int. J. Adv. Res., 2015, 3, 584–590 CAS.
- A. Mroczek, Phytochem. Rev., 2015, 14, 577–605 CrossRef CAS.
- J.-Y. He, N. Ma, S. Zhu, K. Komatsu, Z.-Y. Li and W.-M. Fu, J. Nat. Med., 2015, 69, 1–21 CrossRef CAS PubMed.
- X. Ye, Shijie Nongyao, 2015, 37, 9–14 CAS.
- H.-y. Wang, J. Zhang and T.-h. Xu, Tianran Chanwu Yanjiu Yu Kaifa, 2015, 27, 2142–2148 CAS.
- N. M. Fahmy, E. Al-Sayed and A. N. Singab, Med. Aromat. Plants, 2015, 4, 218 Search PubMed.
- V. V. Grishko, I. A. Tolmacheva and A. V. Pereslavtseva, Chem. Nat. Compd., 2015, 51, 1–21 CrossRef CAS.
- M. Nazir, H. Harms, I. Loef, S. Kehraus, F. El Maddah, I. Arslan, V. Rempel, C. E. Mueller and G. M. König, Planta Med., 2015, 81, 1141–1145 CrossRef CAS PubMed.
- R. B. Williams, V. L. Norman, M. O'Neil-Johnson, S. Woodbury, G. R. Eldridge and C. M. Starks, J. Nat. Prod., 2015, 78, 2074–2086 CrossRef CAS PubMed.
- Z.-M. Chen and S.-L. Wang, Nat. Prod. Res., 2015, 29, 1985–1989 CrossRef CAS PubMed.
- T. Feng, J. He, H.-L. Ai, R. Huang, Z.-H. Li and J.-K. Liu, Nat. Prod. Bioprospect., 2015, 5, 205–208 CrossRef CAS PubMed.
- F. Cen-Pacheco, A. J. Santiago-Benítez, C. Garcia, S. J. Álvarez-Méndez, A. J. Martín-Rodríguez, M. Norte, V. S. Martin, J. A. Gavín, J. J. Fernández and A. H. Daranas, J. Nat. Prod., 2015, 78, 712–721 CrossRef CAS PubMed.
- J.-J. Pan, J. O. Solbiati, G. Ramamoorthy, B. S. Hillerich, R. D. Seidel, J. E. Cronan, S. C. Almo and C. D. Poulter, ACS Cent. Sci., 2015, 1, 77–82 CrossRef CAS PubMed.
- Z.-P. Mai, K. Zhou, G.-B. Ge, C. Wang, X.-K. Huo, P.-P. Dong, S. Deng, B.-J. Zhang, H.-L. Zhang, S.-S. Huang and X.-C. Ma, J. Nat. Prod., 2015, 78, 2372–2380 CrossRef CAS PubMed.
- Z. Liang, T. Zhang, X. Zhang, J. Zhang and C. Zhao, Molecules, 2015, 20, 1424–1433 CrossRef PubMed.
- K. Wang, L. Bao, W. Xiong, K. Ma, J. Han, W. Wang, W. Yin and H. Liu, J. Nat. Prod., 2015, 78, 1977–1989 CrossRef CAS PubMed.
- K. Ma, L. Li, L. Bao, L. He, C. Sun, B. Zhou, S. Si and H. Liu, Tetrahedron, 2015, 71, 1808–1814 CrossRef CAS.
- S.-S. Zhang, Y.-G. Wang, Q.-Y. Ma, S.-Z. Huang, L.-L. Hu, H.-F. Dai, Z.-F. Yu and Y.-X. Zhao, Molecules, 2015, 20, 3281–3289 CrossRef CAS PubMed.
- X. Peng, J. Liu, J. Xia, C. Wang, X. Li, Y. Deng, N. Bao, Z. Zhang and M. Qiu, Phytochemistry, 2015, 114, 137–145 CrossRef CAS PubMed.
- S. Baby, A. J. Johnson and B. Govindan, Phytochemistry, 2015, 114, 66–101 CrossRef CAS PubMed.
- G.-h. Li, Y. Li, X.-l. Mei and J. Lan, Zhongcaoyao, 2015, 46, 1858–1862 CAS.
- S.-s. Huang, S. Deng, H.-l. Zhang, B.-j. Zhang, X.-k. Huo, X.-c. Ma and C. Wang, Zhongguo Weishengtaixue Zazhi, 2015, 27, 1392–1396 CAS.
- X.-R. Zhao, X.-K. Huo, P.-P. Dong, C. Wang, S.-S. Huang, B.-J. Zhang, H.-L. Zhang, S. Deng, K.-X. Liu and X.-C. Ma, J. Nat. Prod., 2015, 78, 1868–1876 CrossRef CAS PubMed.
- Z.-Z. Zhao, R.-H. Yin, H.-P. Chen, T. Feng, Z.-H. Li, Z.-J. Dong, B.-K. Cui and J.-K. Liu, J. Asian Nat. Prod. Res., 2015, 17, 750–755 CrossRef CAS PubMed.
- V. T. Nguyen, N. T. Tung, T. D. Cuong, T. M. Hung, J. A. Kim, M. H. Woo, J. S. Choi, J.-H. Lee and B. S. Min, Phytochem. Lett., 2015, 12, 69–74 CrossRef CAS.
- X.-R. Peng, X. Wang, L. Zhou, B. Hou, Z.-L. Zuo and M.-H. Qiu, RSC Adv., 2015, 5, 95212–95222 RSC.
- W. Feng and J.-S. Yang, J. Asian Nat. Prod. Res., 2015, 17, 1065–1072 CrossRef CAS PubMed.
- T. D. Thang, P.-C. Kuo, T. B. N. Nguyen, T.-L. Hwang, M.-L. Yang, S.-H. Ta, E. J. Lee, D.-H. Kuo, H. H. Nguyen, N. T. Nguyen and T.-S. Wu, J. Nat. Prod., 2015, 78, 2552–2558 CrossRef CAS PubMed.
- X. Yin, Z.-H. Li, Y. Li, T. Feng and J.-K. Liu, J. Asian Nat. Prod. Res., 2015, 17, 793–799 CrossRef CAS PubMed.
- P. Pimjuk, C. Phosri, T. Wauke and S. McCloskey, Phytochem. Lett., 2015, 14, 79–83 CrossRef CAS.
- S.-S. Zhang, Q.-Y. Ma, S.-Z. Huang, H.-F. Dai, Z.-K. Guo, Z.-F. Yu and Y.-X. Zhao, Phytochemistry, 2015, 110, 133–139 CrossRef CAS PubMed.
- G. Li, S. Kusari, P. Kusari, O. Kayser and M. Spiteller, J. Nat. Prod., 2015, 78, 2128–2132 CrossRef CAS PubMed.
- T. Suga, Y. Asami, S. Hashimoto, K. Nonaka, M. Iwatsuki, T. Nakashima, R. Sugahara, T. Shiotsuki, T. Yamamoto, Y. Shinohara, N. Ichimaru, M. Murai, H. Miyoshi, S. Omura and K. Shiomi, J. Antibiot., 2015, 68, 649–652 CrossRef CAS PubMed.
- J.-J. Han, L. Bao, Q.-Q. Tao, Y.-J. Yao, X.-Z. Liu, W.-B. Yin and H.-W. Liu, Org. Lett., 2015, 17, 2538–2541 CrossRef CAS PubMed.
- F. Cateni, V. Lucchini, M. Zacchigna, G. Procida, B. Doljak and M. Anderluh, Chem. Nat. Compd., 2015, 51, 74–80 CrossRef CAS.
- F. Zhao, Q. Mai, J. Ma, M. Xu, X. Wang, T. Cui, F. Qiu and G. Han, Fitoterapia, 2015, 101, 34–40 CrossRef CAS PubMed.
- F.-C. Ren, L.-X. Wang, Q. Yu, X.-J. Jiang and F. Wang, Nat. Prod. Bioprospect., 2015, 5, 263–270 CrossRef CAS PubMed.
- J. K. Sihra, A. E. Thumser, M. K. Langat, N. R. Crouch and D. A. Mulholland, Nat. Prod. Commun., 2015, 10, 1207–1209 CrossRef PubMed.
- Q.-Q. Zhao, Q.-Y. Song, K. Jiang, G.-D. Li, W.-J. Wei, Y. Li and K. Gao, Org. Lett., 2015, 17, 2760–2763 CrossRef CAS PubMed.
- G.-W. Wang, C. Lv, X. Yuan, J. Ye, H.-Z. Jin, L. Shan, X.-K. Xu, Y.-H. Shen and W.-D. Zhang, Phytochemistry, 2015, 116, 221–229 CrossRef CAS PubMed.
- S. Lavoie, C. Gauthier, V. Mshvildadze, J. Legault, B. Roger and A. Pichette, J. Nat. Prod., 2015, 78, 2896–2907 CrossRef CAS PubMed.
- C.-Q. Liang, Y.-M. Shi, W.-G. Wang, Z.-X. Hu, Y. Li, Y.-T. Zheng, X.-N. Li, X. Du, J.-X. Pu, W.-L. Xiao, H.-B. Zhang and H.-D. Sun, J. Nat. Prod., 2015, 78, 2067–2073 CrossRef CAS PubMed.
- Z.-X. Hu, Y.-M. Shi, W.-G. Wang, X.-N. Li, X. Du, M. Liu, Y. Li, Y.-B. Xue, Y.-H. Zhang, J.-X. Pu and H.-D. Sun, Org. Lett., 2015, 17, 4616–4619 CrossRef CAS PubMed.
- D.-x. Li, J. Li, Y.-p. Yin and L.-j. Xuan, Chin. Herb. Med., 2015, 7, 283–286 CrossRef CAS.
- E. G. Lyakhova, S. A. Kolesnikova, A. I. Kalinovsky, P. S. Dmitrenok, N. H. Nam and V. A. Stonik, Steroids, 2015, 96, 37–43 CrossRef CAS PubMed.
- J.-K. Woo, C.-K. Kim, C.-H. Ahn, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2015, 78, 218–224 CrossRef CAS PubMed.
- G. Genta-Jouve, C. Boughanem, O. Ocaña, T. Pérez and O. P. Thomas, Phytochem. Lett., 2015, 13, 252–255 CrossRef CAS.
- S. M. Mohamed, E. Y. Bachkeet, S. A. Bayoumi, S. Jain, S. J. Cutler, B. L. Tekwani and S. A. Ross, Fitoterapia, 2015, 107, 114–121 CrossRef CAS PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko, I. Y. Dolmatov and V. I. Kalinin, Carbohydr. Res., 2015, 414, 22–31 CrossRef CAS PubMed.
- Y. Bahrami and C. M. M. Franco, Mar. Drugs, 2015, 13, 597–617 CrossRef PubMed.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, V. I. Kalinin, E. A. Yurchenko and I. Y. Dolmatov, Nat. Prod. Commun., 2015, 10, 1687–1694 Search PubMed.
- A. I. Kalinovsky, A. S. Silchenko, S. A. Avilov and V. I. Kalinin, Nat. Prod. Commun., 2015, 10, 1167–1170 CrossRef PubMed.
- N. X. Cuong, L. T. Vien, T. T. H. Hanh, N. P. Thao, D. T. Thao, N. V. Thanh, N. H. Nam, D. C. Thung, P. V. Kiem and C. V. Minh, Bioorg. Med. Chem. Lett., 2015, 25, 3151–3156 CrossRef CAS PubMed.
- A. S. Silchenko, A. I. Kalinovsky, P. S. Dmitrenok, V. I. Kalinin, A. N. Mazeika, N. S. Vorobieva, N. M. Sanina and E. Y. Kostetsky, Nat. Prod. Commun., 2015, 10, 877–880 Search PubMed.
- S. Yu, X. Ye, H. Huang, R. Peng, Z. Su, X.-Y. Lian and Z. Zhang, Planta Med., 2015, 81, 152–159 CrossRef CAS PubMed.
- M. Honey-Escandón, R. Arreguín-Espinosa, F. A. Solis-Marín and Y. Samyn, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2015, 180, 16–39 CrossRef PubMed.
- N. B. Janakiram, A. Mohammed and C. V. Rao, Mar. Drugs, 2015, 13, 2909–2923 CrossRef CAS PubMed.
- Y. Nian, J. Yang, T.-Y. Liu, Y. Luo, J.-H. Zhang and M.-H. Qiu, Sci. Rep., 2015, 5, 9026 CrossRef CAS PubMed.
- J.-H. Yang, J.-X. Pu, J. Wen, X.-N. Li, F. He, J. Su, Y. Li and H.-D. Sun, Phytochemistry, 2015, 109, 36–42 CrossRef CAS PubMed.
- G.-W. Wang, C. Lv, X. Fang, X.-H. Tian, J. Ye, H.-L. Li, L. Shan, Y.-H. Shen and W.-D. Zhang, J. Nat. Prod., 2015, 78, 50–60 CrossRef CAS PubMed.
- D.-J. Zheng, J. Zhou, Q. Liu, W. Yao, M.-Z. Zhang, B.-H. Shao, J.-X. Mo, C.-X. Zhou and L.-S. Gan, Fitoterapia, 2015, 103, 283–288 CrossRef CAS PubMed.
- L.-S. Gan, D.-J. Zheng, Q. Liu, J. Zhou, M.-Z. Zhang, W. Yao, B.-H. Shao, J.-X. Mo and C.-X. Zhou, Bioorg. Med. Chem. Lett., 2015, 25, 3845–3849 CrossRef CAS PubMed.
- K. Jiang, L.-L. Chen, S.-F. Wang, Y. Wang, Y. Li and K. Gao, J. Nat. Prod., 2015, 78, 1037–1044 CrossRef CAS PubMed.
- Z.-B. Wang, Y.-D. Zhai, Z.-P. Ma, C.-J. Yang, R. Pan, J.-L. Yu, Q.-H. Wang, B.-Y. Yang and H.-X. Kuang, Chem. Biodiversity, 2015, 12, 1575–1584 CrossRef CAS PubMed.
- T. Mayanti, J. Sianturi, D. Harneti, Darwati, U. Supratman, M. M. Rosli and H.-K. Fun, Molbank, 2015, M880, DOI:10.3390/m880.
- M. Satiraphan, Q. D. Thai, U. Sotanaphun, C. Sittisombut, S. Michel and X. Cachet, Nat. Prod. Res., 2015, 29, 1820–1827 CrossRef CAS PubMed.
- K. Panthong, R. Sompong, V. Rukachaisirikul, N. Hutadilok-Towatana, S. P. Voravuthikunchai and J. Saising, Phytochem. Lett., 2015, 11, 43–48 CrossRef CAS.
- G. Gilardoni, X. Chiriboga, P. Vita Finzi and G. Vidari, Chem. Biodiversity, 2015, 12, 946–954 CrossRef CAS PubMed.
- L. Maamria, C. Long, H. Haba, C. Lavaud, A. Cannac and M. Benkhaled, Phytochem. Lett., 2015, 11, 286–291 CrossRef CAS.
- L. Wu, Z.-l. Chen, Y. Su, Q.-h. Wang and H.-x. Kuang, Zhongguo Tianran Yaowu, 2015, 13, 81–89 CAS.
- N.-M. Bao, Y. Nian, W.-H. Wang, X.-L. Liu, Z.-T. Ding and M.-H. Qiu, Phytochem. Lett., 2015, 12, 200–202 CrossRef CAS.
- W.-H. Wang, Y. Nian, Y.-J. He, L.-S. Wan, N.-M. Bao, G.-L. Zhu, F. Wang and M.-h. Qiu, Tetrahedron, 2015, 71, 8018–8025 CrossRef CAS.
- M. Liu, Y.-Q. Luo, W.-G. Wang, Y.-M. Shi, H.-Y. Wu, X. Du, J.-X. Pu and H.-D. Sun, Nat. Prod. Commun., 2015, 10, 2045–2047 Search PubMed.
- Y.-G. Xia, B.-Y. Yang and H.-X. Kuang, Phytochem. Rev., 2015, 14, 155–187 CrossRef CAS.
- Y.-M. Shi, W.-L. Xiao, J.-X. Pu and H.-D. Sun, Nat. Prod. Rep., 2015, 32, 367–410 RSC.
- M. N. Samy, H. E. Khalil, S. Sugimoto, K. Matsunami, H. Otsuka and M. S. Kamel, Phytochemistry, 2015, 115, 261–268 CrossRef CAS PubMed.
- D.-F. Zhu, G.-L. Zhu, L.-M. Kong, N.-M. Bao, L. Zhou, Y. Nian and M.-H. Qiu, Nat. Prod. Bioprospect., 2015, 5, 61–67 CrossRef CAS PubMed.
- U. Kaushik, V. Aeri and S. R. Mir, Pharmacogn. Rev., 2015, 9, 12–18 CrossRef CAS PubMed.
- Y. Cai, X. Fang, C. He, P. Li, F. Xiao, Y. Wang and M. Chen, Am. J. Chin. Med., 2015, 43, 1331–1350 CrossRef CAS PubMed.
- E. Attard and M.-G. Martinoli, Curr. Top. Med. Chem., 2015, 15, 1708–1713 CrossRef CAS PubMed.
- L. Dai, C. Liu, Y. Zhu, J. Zhang, Y. Men, Y. Zeng and Y. Sun, Plant Cell Physiol., 2015, 56, 1172–1182 CrossRef CAS PubMed.
- C.-C. Liaw, H.-C. Huang, P.-C. Hsiao, L.-J. Zhang, Z.-H. Lin, S.-Y. Hwang, F.-L. Hsu and Y.-H. Kuo, Planta Med., 2015, 81, 62–70 CAS.
- J.-C. Chen, C. B.-S. Lau, J. Y.-W. Chan, K.-P. Fung, P.-C. Leung, J.-Q. Liu, L. Zhou, M.-J. Xie and M.-H. Qiu, Planta Med., 2015, 81, 327–332 CrossRef CAS PubMed.
- Z.-J. Li, J.-C. Chen, Y.-Y. Deng, N.-L. Song, M.-Y. Yu, L. Zhou and M.-H. Qiu, Helv. Chim. Acta, 2015, 98, 1456–1461 CrossRef CAS.
- Y. He, Q. Shang and L. Tian, J. Chem. Res., 2015, 39, 70–72 CrossRef CAS.
- Y. Li, Z. Zheng, L. Zhou, Y. Liu, H. Wang, L. Li and Q. Yao, Phytochem. Lett., 2015, 14, 239–244 CrossRef CAS.
- R. Chawech, R. Jarraya, C. Girardi, M. Vansteelandt, G. Marti, I. Nasri, C. Racaud-Sultan and N. Fabre, Molecules, 2015, 20, 18001–18015 CrossRef CAS PubMed.
- F. Song, B. Dai, H.-Y. Zhang, J.-W. Xie, C.-Z. Gu and J. Zhang, J. Asian Nat. Prod. Res., 2015, 17, 813–818 CrossRef CAS PubMed.
- V. S. P. Chaturvedula and S. R. Meneni, Nat. Prod. Commun., 2015, 10, 1521–1523 CrossRef PubMed.
- J. Cao, X. Zhang, F. Qu, Z. Guo and Y. Zhao, Expert Opin. Ther. Pat., 2015, 25, 805–817 CrossRef CAS PubMed.
- B.-K. Shin, S. W. Kwon and J. H. Park, J. Ginseng Res., 2015, 39, 287–298 CrossRef CAS PubMed.
- Y. Wang, Y. Chu, W. Li, X.-h. Ma and Z.-p. Wei, Zhongcaoyao, 2015, 46, 1381–1392 CAS.
- J. Li, R.-f. Wang, L. Yang and Z.-t. Wang, Zhongguo Zhongyao Zazhi, 2015, 40, 3480–3487 CAS.
- Q.-L. Zhou and X.-W. Yang, Bioorg. Med. Chem. Lett., 2015, 25, 3112–3116 CrossRef CAS PubMed.
- L.-Y. Ma and X.-W. Yang, Phytochem. Lett., 2015, 13, 406–412 CrossRef CAS.
- C.-Z. Gu, J.-J. Lv, X.-X. Zhang, Y.-J. Qiao, H. Yan, Y. Li, D. Wang, H.-T. Zhu, H.-R. Luo, C.-R. Yang, M. Xu and Y.-J. Zhang, J. Nat. Prod., 2015, 78, 1829–1840 CrossRef CAS PubMed.
- C.-Z. Gu, J.-J. Lv, X.-X. Zhang, H. Yan, H.-T. Zhu, H.-R. Luo, D. Wang, C.-R. Yang, M. Xu and Y.-J. Zhang, Fitoterapia, 2015, 103, 97–105 CrossRef CAS PubMed.
- X.-S. Zhang, J.-Q. Cao, C. Zhao, X.-d. Wang, X.-j. Wu and Y.-Q. Zhao, Bioorg. Med. Chem. Lett., 2015, 25, 3095–3099 CrossRef CAS PubMed.
- B.-S. Cui and S. Li, Chin. Chem. Lett., 2015, 26, 585–589 CrossRef CAS.
- P. T. Anh, P. T. Ky, N. T. Cue, N. X. Nhiem, P. H. Yen, T. M. Ngoc, H. L. T. Anh, B. H. Tai, D. T. Trang, C. V. Minh and P. V. Kiem, Nat. Prod. Commun., 2015, 10, 1351–1352 CrossRef PubMed.
- Y. Zhou, B. Yang, Z. Liu, Y. Jiang, Y. Liu, L. Fu, X. Wang and H. Kuang, Molecules, 2015, 20, 19252–19262 CrossRef CAS PubMed.
- T. Pathomwichaiwat, P. Ochareon, N. Soonthornchareonnon, Z. Ali, I. A. Khan and S. Prathanturarug, J. Ethnopharmacol., 2015, 160, 52–60 CrossRef CAS PubMed.
- J. Hu, H. Li, B.-S. Yang, X. Mao and X.-D. Shi, Helv. Chim. Acta, 2015, 98, 273–278 CrossRef CAS.
- X.-Q. Chen, L.-D. Shao, M. Pal, Y. Shen, X. Cheng, G. Xu, L.-Y. Peng, K. Wang, Z.-H. Pan, M.-M. Li, Y. Leng, J. He and Q.-S. Zhao, J. Nat. Prod., 2015, 78, 330–334 CrossRef CAS PubMed.
- C. Yuan, F.-X. Xu, S.-P. Li, X.-J. Huang and Q.-W. Zhang, Chin. J. Nat. Med., 2015, 13, 303–306 CAS.
- J.-q. Cao, W. Li, Y. Tang, X.-r. Zhang and Y.-q. Zhao, Phytochem. Lett., 2015, 11, 301–305 CrossRef CAS.
- X.-w. Yang, K.-k. Li and Q.-l. Zhou, Zhongcaoyao, 2015, 46, 3137–3145 CAS.
- C. Lee, J. W. Lee, Q. Jin, H. Jang, H.-J. Jang, M.-C. Rho, M. K. Lee, C. K. Lee, M. K. Lee and B. Y. Hwang, J. Nat. Prod., 2015, 78, 971–976 CrossRef CAS PubMed.
- K.-k. Li and X.-w. Yang, Zhongcaoyao, 2015, 46, 169–173 CAS.
- L.-Y. Ma, Q.-L. Zhou and X.-W. Yang, Bioorg. Med. Chem. Lett., 2015, 25, 5321–5325 CrossRef CAS PubMed.
- D. G. Lee, A. Y. Lee, K.-T. Kim, E. J. Cho and S. Lee, Chem. Pharm. Bull., 2015, 63, 927–934 CrossRef CAS PubMed.
- Y. Liu, L. Cheng, J.-h. Yeon, Q.-q. He and D.-y. Kong, Zhongcaoyao, 2015, 46, 1251–1258 CAS.
- J.-H. Yu, Y. Shen, Y. Wu, Y. Leng, H. Zhang and J.-M. Yue, RSC Adv., 2015, 5, 26777–26784 RSC.
- J.-J. Ge, P. Chen and X.-X. Ye, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2015, 71, o464–o465 CrossRef CAS PubMed.
- Q. Zhu, L. Lin, C. Tang, C. Ke and Y. Ye, Rec. Nat. Prod., 2015, 9, 267–270 Search PubMed.
- F.-H. Fang, W.-H. Li, Z.-Z. Han, W.-J. Huang, D.-X. Li, S. Zhao, M.-H. Tang and C.-S. Yuan, J. Asian Nat. Prod. Res., 2015, 17, 1213–1219 CrossRef CAS PubMed.
- C. H. Miguita, C. d. S. Barbosa, L. Hamerski, U. C. Sarmento, J. Nicacio do Nascimento, W. S. Garcez and F. R. Garcez, Molecules, 2015, 20, 111–126 CrossRef CAS PubMed.
- G. M. Happi, S. F. Kouam, F. M. Talontsi, S. Zühlke, M. Lamshöft and M. Spiteller, Fitoterapia, 2015, 102, 35–40 CrossRef CAS PubMed.
- E. A. Mireku, S. Kusari, D. Eckelmann, A. Y. Mensah, F. M. Talontsi and M. Spiteller, Fitoterapia, 2015, 106, 84–91 CrossRef CAS PubMed.
- L. Jing, Y.-M. Zhang, J.-G. Luo and L.-Y. Kong, Chem. Pharm. Bull., 2015, 63, 237–243 CrossRef CAS PubMed.
- L. Misra, S. Gupta and R. S. Sangwan, J. Indian Chem. Soc., 2015, 92, 111–114 CAS.
- G. M. Happi, S. F. Kouam, F. M. Talontsi, M. Lamshöft, S. Zühlke, J. O. Bauer, C. Strohmann and M. Spiteller, J. Nat. Prod., 2015, 78, 604–614 CrossRef CAS PubMed.
- H. T. P. Nguyen, T. D. T. Nguyen, N. T. Ngoc, P. T. Nguyen, S. Kim, Y. S. Koh, V. T. Nguyen, X. C. Nguyen, H. N. Nguyen, V. K. Phan, Y. H. Kim and V. M. Chau, Chem. Pharm. Bull., 2015, 63, 558–564 CrossRef CAS PubMed.
- J.-L. Giner and T. N. Schroeder, Chem. Biodiversity, 2015, 12, 1126–1129 CrossRef CAS PubMed.
- M. Suri Appa Rao, G. Suresh, P. Ashok Yadav, K. Rajendra Prasad, P. Usha Rani, C. Venkata Rao and K. Suresh Babu, Tetrahedron, 2015, 71, 1431–1437 CrossRef CAS.
- Y.-B. Wu, D. Liu, P.-Y. Liu, X.-M. Yang, M. Liao, N.-N. Lu, F. Sauriol, Y.-C. Gu, Q.-W. Shi, H. Kiyota and M. Dong, Helv. Chim. Acta, 2015, 98, 691–698 CrossRef CAS.
- N. Kim, K.-W. Cho, S. S. Hong, B. Y. Hwang, T. Chun and D. Lee, Bioorg. Med. Chem. Lett., 2015, 25, 621–625 CrossRef CAS PubMed.
- A.-R. Choi, I.-K. Lee, E. E. Woo, J.-W. Kwon, B.-S. Yun and H.-R. Park, Chem. Pharm. Bull., 2015, 63, 1065–1069 CrossRef CAS PubMed.
- Z.-K. Guo, T. Yang, C.-H. Cai, W.-H. Dong, C.-J. Gai, J.-Z. Yuan, W.-L. Mei and H.-F. Dai, J. Asian Nat. Prod. Res., 2015, 17, 1018–1023 CrossRef CAS PubMed.
- M.-L. Han, J.-X. Zhao, H.-C. Liu, G. Ni, J. Ding, S.-P. Yang and J.-M. Yue, J. Nat. Prod., 2015, 78, 754–761 CrossRef CAS PubMed.
- Y.-Y. Fan, H. Zhang, Y. Zhou, H.-B. Liu, W. Tang, B. Zhou, J.-P. Zuo and J.-M. Yue, J. Am. Chem. Soc., 2015, 137, 138–141 CrossRef CAS PubMed.
- Y. Ren, C. Yuan, Y. Deng, R. Kanagasabai, T. N. Ninh, V. T. Tu, H.-B. Chai, D. D. Soejarto, J. R. Fuchs, J. C. Yalowich, J. Yu and A. Douglas Kinghorn, Phytochemistry, 2015, 111, 132–140 CrossRef CAS PubMed.
- B. Zhou, Y. Shen, Y. Wu, Y. Leng and J.-M. Yue, J. Nat. Prod., 2015, 78, 2116–2122 CrossRef CAS PubMed.
- K.-L. Ji, P. Zhang, X.-N. Li, J. Guo, H.-B. Hu, C.-F. Xiao, X.-Q. Xie and Y.-K. Xu, Phytochemistry, 2015, 118, 61–67 CrossRef CAS PubMed.
- Y. Yan, J.-X. Zhang, T. Huang, X.-Y. Mao, W. Gu, H.-P. He, Y.-T. Di, S.-L. Li, D.-Z. Chen, Y. Zhang and X.-J. Hao, J. Nat. Prod., 2015, 78, 811–821 CrossRef CAS PubMed.
- Y. Yan, C.-M. Yuan, Y.-T. Di, T. Huang, Y.-M. Fan, Y. Ma, J.-X. Zhang and X.-J. Hao, Fitoterapia, 2015, 107, 29–35 CrossRef CAS PubMed.
- M.-S. Yang, S.-M. Hu, L.-Y. Kong and J. Luo, Tetrahedron, 2015, 71, 8472–8477 CrossRef CAS.
- Y.-X. Yan, J.-Q. Liu, H.-W. Wang, J.-X. Chen, J.-C. Chen, L. Chen, L. Zhou and M.-H. Qiu, Chem. Biodiversity, 2015, 12, 1040–1046 CrossRef CAS PubMed.
- Y.-X. Yan, J.-Q. Liu, J.-X. Chen, J.-C. Chen and M.-H. Qiu, J. Asian Nat. Prod. Res., 2015, 17, 14–19 CrossRef CAS PubMed.
- I. Dilshad, B. S. Siddiqui and S. Faizi, Helv. Chim. Acta, 2015, 98, 135–142 CrossRef CAS.
- R. G. Fuentes, K. Toume, M. A. Arai, S. K. Sadhu, F. Ahmed and M. Ishibashi, Phytochem. Lett., 2015, 11, 280–285 CrossRef CAS.
- J.-H. Yu, G.-C. Wang, Y.-S. Han, Y. Wu, M. A. Wainberg and J.-M. Yue, J. Nat. Prod., 2015, 78, 1243–1252 CrossRef CAS PubMed.
- W. Li, Z. Jiang, L. Shen, P. Pedpradab, T. Bruhn, J. Wu and G. Bringmann, J. Nat. Prod., 2015, 78, 1570–1578 CrossRef CAS PubMed.
- K. Shao, L. Shen and J. Wu, Zhongcaoyao, 2015, 46, 2198–2205 CAS.
- L.-C. Chen, H.-R. Liao, P.-Y. Chen, W.-L. Kuo, T.-H. Chang, P.-J. Sung, Z.-H. Wen and J.-J. Chen, Molecules, 2015, 20, 18551–18564 CrossRef CAS PubMed.
- B. Ovalle-Magallanes, O. N. Medina-Campos, J. Pedraza-Chaverri and R. Mata, Phytochemistry, 2015, 110, 111–119 CrossRef CAS PubMed.
- Y. Li, Q. Lu, J. Luo, J. Wang, X. Wang, M. Zhu and L. Kong, Chem. Pharm. Bull., 2015, 63, 305–310 CrossRef PubMed.
- H. Li, Y. Li, X.-B. Wang, T. Pang, L.-Y. Zhang, J. Luo and L.-Y. Kong, RSC Adv., 2015, 5, 40465–40474 RSC.
- A. Sakamoto, Y. Tanaka, T. Yamada, T. Kikuchi, O. Muraoka, K. Ninomiya, T. Morikawa and R. Tanaka, Fitoterapia, 2015, 100, 81–87 CrossRef CAS PubMed.
- T. Miyake, S. Ishimoto, N. Ishimatsu, K. Higuchi, K. Minoura, T. Kikuchi, T. Yamada, O. Muraoka and R. Tanaka, Molecules, 2015, 20, 20955–20966 CrossRef CAS PubMed.
- T. Inoue, Y. Matsui, T. Kikuchi, T. Yamada, Y. In, O. Muraoka, C. Sakai, K. Ninomiya, T. Morikawa and R. Tanaka, Tetrahedron, 2015, 71, 2753–2760 CrossRef CAS.
- H. Li, J. Luo and L. Kong, Rec. Nat. Prod., 2015, 9, 190–195 Search PubMed.
- W.-X. Liu, D.-Z. Chen, J.-Y. Ding, X.-J. Hao and S.-L. Li, Helv. Chim. Acta, 2015, 98, 1403–1410 CrossRef CAS.
- J. Luo, H.-J. Zhang, O. Quasie, S.-M. Shan, Y.-M. Zhang and L.-Y. Kong, Phytochemistry, 2015, 117, 410–416 CrossRef CAS PubMed.
- X. Fang, Y. T. Di, Y. Zhang, Z. P. Xu, Y. Lu, Q. Q. Chen, Q. T. Zheng and X. J. Hao, Angew. Chem., Int. Ed., 2015, 54, 5592–5595 CrossRef CAS PubMed.
- Q.-M. Ye, L.-L. Bai, S.-Z. Hu, H.-Y. Tian, L.-J. Ruan, Y.-F. Tan, L.-P. Hu, W.-C. Ye, D.-M. Zhang and R.-W. Jiang, Fitoterapia, 2015, 105, 66–72 CrossRef CAS PubMed.
- N. N. Win, T. Ito, Ismail, T. Kodama, Y. Y. Win, M. Tanaka, H. Ngwe, Y. Asakawa, I. Abe and H. Morita, J. Nat. Prod., 2015, 78, 3024–3030 CrossRef CAS PubMed.
- M. Masaoud, K. Hussein, A. H. Ahmed and M. A. Al Maqtari, World J. Pharm. Pharm. Sci., 2015, 4, 182–194 CAS.
- Y. Selim, Nat. Prod.: Indian J., 2015, 11, 90–96 Search PubMed.
- Y. Selim and K. Litinas, Med. Chem. Res., 2015, 24, 4016–4022 CrossRef CAS.
- Y. A. Selim and K. E. Litinas, J. Chil. Chem. Soc., 2015, 60, 2896–2899 CrossRef CAS.
- O. Callies, L. M. Bedoya, M. Beltrán, A. Muñoz, P. O. Calderón, A. A. Osorio, I. A. Jiménez, J. Alcamí and I. L. Bazzocchi, J. Nat. Prod., 2015, 78, 1045–1055 CrossRef CAS PubMed.
- Harizon, B. Pujiastuti, D. Kurnia, D. Sumiarsa, Y. Shiono and U. Supratman, Nat. Prod. Commun., 2015, 10, 277–280 CrossRef CAS PubMed.
- F. A. A. Toze, M. Fomani, A. B. Nouga, J. R. Chouna, Kouam, A. F. K. Waffo and J. D. Wansi, Int. Res. J. Pure Appl. Chem., 2015, 7, 157–164 CrossRef CAS.
- Q.-Y. Liu, F. Wang, L. Zhang, J.-M. Xie, P. Li and Y.-H. Zhang, Helv. Chim. Acta, 2015, 98, 627–632 CrossRef CAS.
- N. Jackson, K. Annan, A. Y. Mensah, E. Ekuadzi, M. L. K. Mensah and S. Habtemariam, Nat. Prod. Commun., 2015, 10, 563–564 CrossRef PubMed.
- Y.-M. Ying, C.-Y. Li, Y. Chen, J.-G. Xiang, L. Fang, J.-B. Yao, F.-S. Wang, R.-W. Wang, W.-G. Shan and Z.-J. Zhan, Chem. Biodiversity, 2015, 12, 1222–1228 CrossRef CAS PubMed.
- Z. Chen, L. Tong, Y. Feng, J. Wu, X. Zhao, H. Ruan, H. Pi and P. Zhang, Phytochemistry, 2015, 116, 329–336 CrossRef CAS PubMed.
- I. A. Oladosu, S. O. Balogun and Z.-Q. Liu, Chin. J. Nat. Med., 2015, 13, 133–141 CAS.
- S. Ahmad, R. Ullah, N. M. AbdEl-Salam, M. Arfan and H. Hussain, J. Asian Nat. Prod. Res., 2015, 17, 838–842 CrossRef CAS PubMed.
- A. P. Singh and S. K. Sharma, Hygeia, 2015, 7, 1–9 CAS.
- J.-F. Xu, H.-B. Wu, D.-C. Liu, L. Sha, W.-H. Wu, H. Fan, Y.-S. Song and H.-G. Zhu, Bull. Korean Chem. Soc., 2015, 36, 2862–2868 CrossRef CAS.
- H.-b. Wu, D.-c. Liu and J.-f. Xu, Tianran Chanwu Yanjiu Yu Kaifa, 2015, 27, 18–21 CAS.
- C. Chen, G. Wei, H. Zhu, Y. Guo, X.-N. Li, J. Zhang, Y. Liu, G. Yao, Z. Luo, Y. Xue and Y. Zhang, Fitoterapia, 2015, 103, 227–230 CrossRef CAS PubMed.
- A. Qiao, Y. Wang, L. Xiang, Z. Zhang and X. He, J. Funct. Foods, 2015, 13, 308–313 CrossRef CAS.
- D.-l. Meng, L.-h. Xu, C. Chen, D. Yan, Z.-z. Fang and Y.-f. Cao, J. Funct. Foods, 2015, 16, 28–39 CrossRef CAS.
- Z.-J. Lia, C.-P. Wan, L. Cai, S.-Q. Li, X. Zheng, Y. Qi, J.-W. Dong, T.-P. Yin, Z.-X. Zhou, N.-H. Tan and Z.-T. Ding, Phytochem. Lett., 2015, 13, 246–251 CrossRef CAS.
- Y. Ren, A. Van Schoiack, H.-B. Chai, M. Goetz and A. D. Kinghorn, J. Nat. Prod., 2015, 78, 2440–2446 CrossRef CAS PubMed.
- S. Begum, A. Ayub, B. Shaheen Siddiqui, S. Fayyaz and F. Kazi, Chem. Biodiversity, 2015, 12, 1435–1442 CrossRef CAS PubMed.
- C. Zhang, K. Jiang, S.-J. Qu, Y.-M. Zhai, J.-J. Tan and C.-H. Tan, J. Asian Nat. Prod. Res., 2015, 17, 996–1001 CrossRef CAS PubMed.
- K. Polatoğlu and N. Gören, Rec. Nat. Prod., 2015, 9, 419–422 Search PubMed.
- F. Wang, Y.-B. Wang, H. Chen, L. Chen, S.-W. Liang and S.-M. Wang, J. Asian Nat. Prod. Res., 2015, 17, 823–827 CrossRef CAS PubMed.
- C. Yin, J. Zhou, Y. Wu, Y. Cao, T. Wu, S. Zhang, H. Li and Z. Cheng, Fitoterapia, 2015, 106, 46–54 CrossRef CAS PubMed.
- S. Srisurichan and S. Pornpakakul, Phytochem. Lett., 2015, 12, 282–286 CrossRef CAS.
- Y. Luo, Q.-L. Xu, L.-M. Dong, Z.-Y. Zhou, Y.-C. Chen, W.-M. Zhang and J.-W. Tan, Phytochem. Lett., 2015, 11, 127–131 CrossRef CAS.
- J. Wang, H. Ren, Q.-L. Xu, Z.-Y. Zhou, P. Wu, X.-Y. Wei, Y. Cao, X.-X. Chen and J.-W. Tan, Food Chem., 2015, 168, 623–629 CrossRef CAS PubMed.
- D. Yang, D. Guo, Y. Zhou, W. Mei and S. Xiao, Youji Huaxue, 2015, 35, 1781–1784 CrossRef CAS.
- R. N. Ache, T. K. Tabopda, S. O. Yeboah and B. T. Ngadjui, Nat. Prod. Commun., 2015, 10, 1933–1936 CrossRef.
- Q. Wu, G.-Z. Tu and H.-Z. Fu, Magn. Reson. Chem., 2015, 53, 544–550 CrossRef CAS PubMed.
- Y.-F. Zheng, J.-H. Wei, S.-Q. Fang, Y.-P. Tang, H.-B. Cheng, T.-L. Wang, C.-Y. Li and G.-P. Peng, Molecules, 2015, 20, 6273–6283 CrossRef CAS PubMed.
- J. Leng, Y.-x. Zhu, L.-l. Chen and S.-f. Wang, Zhongcaoyao, 2015, 46, 1576–1582 CAS.
- B. Ali, R. Tabassum, N. Riaz, A. Yaqoob, T. Khatoon, R. B. Tareen, A. Jabbar, F.-u.-H. Nasim and M. Saleem, J. Asian Nat. Prod. Res., 2015, 17, 843–850 CrossRef CAS PubMed.
- R. Farheen, B. S. Siddiqui, I. Mahmood, S. U. Simjee and S. Majeed, Phytochem. Lett., 2015, 13, 256–261 CrossRef CAS.
- C. Wu, R.-L. Zhang, H.-Y. Li, C. Hu, B.-L. Liu, Y.-L. Li and G.-X. Zhou, Carbohydr. Res., 2015, 413, 107–114 CrossRef CAS PubMed.
- C.-N. Lv, L. Fan, J. Wang, R.-L. Qin, T.-Y. Xu, T.-L. Lei and J.-C. Lu, J. Asian Nat. Prod. Res., 2015, 17, 132–137 CrossRef CAS PubMed.
- D.-Q. Li, J. Wu, L.-Y. Liu, Y.-Y. Wu, L.-Z. Li, X.-X. Huang, Q.-B. Liu, J.-Y. Yang, S.-J. Song and C.-F. Wu, Bioorg. Med. Chem. Lett., 2015, 25, 3887–3892 CrossRef CAS PubMed.
- M.-L. Wang, Y.-L. Liu, Q.-M. Xu, Y.-Q. Li and S.-L. Yang, J. Asian Nat. Prod. Res., 2015, 17, 908–914 CrossRef CAS PubMed.
- A. J. Pérez, E. M. Hassan, L. Pecio, E. A. Omer, M. Kucinska, M. Murias and A. Stochmal, Phytochem. Lett., 2015, 13, 59–67 CrossRef.
- W. Yuan, P. Wang, Z. Su, R. Gao and S. Li, Phytochem. Lett., 2015, 14, 111–114 CrossRef CAS.
- H. J. Ko, J. H. Lee, Y. S. Kim, J. H. Lee and E.-R. Woo, Bull. Korean Chem. Soc., 2015, 36, 356–359 CrossRef CAS.
- E. A. Ragab, Med. Chem. Res., 2015, 24, 2916–2925 CrossRef CAS.
- T. H. V. Nguyen, T. A. Vien, P. V. Kiem, C. V. Minh, X. N. Nguyen, P. Q. Long, L. T. Anh, N. Kim, S. J. Park and S. H. Kim, Arch. Pharmacal Res., 2015, 38, 1926–1931 CrossRef PubMed.
- F. Guan, Q. Wang, M. Wang, Y. Shan, Y. Chen, M. Yin, Y. Zhao, X. Feng, F. Liu and J. Zhang, Molecules, 2015, 20, 6419–6431 CrossRef CAS PubMed.
- Y. Shan, H. Li, F. Guan, Y. Chen, M. Yin, M. Wang, X. Feng and Q. Wang, Molecules, 2015, 20, 20334–20340 CrossRef CAS PubMed.
- O. P. Noté, D. Jihu, C. Antheaume, D. Guillaume, D. E. Pegnyemb, M. C. Kilhoffer and A. Lobstein, Phytochem. Lett., 2015, 11, 37–42 CrossRef.
- J. Li, X. Huang, X.-H. Jiang, Q.-F. Zhu, Y. Yang and G.-C. Gao, Chem. Pharm. Bull., 2015, 63, 388–392 CrossRef CAS PubMed.
- Y. Shao, D.-W. Ou-Yang, L. Cheng, W. Gao, X.-X. Weng and D.-Y. Kong, Helv. Chim. Acta, 2015, 98, 683–690 CrossRef CAS.
- R. Rattan, S. G. E. Reddy, S. K. Dolma, B. I. Fozdar, V. Gautam, R. Sharma and U. Sharma, Nat. Prod. Commun., 2015, 10, 1525–1528 CrossRef PubMed.
- Y. Yuan, Y.-Q. Qiao, B.-S. Cui, L. Tang, S.-P. Yuan, Q. Hou and S. Li, J. Asian Nat. Prod. Res., 2015, 17, 239–247 CrossRef CAS PubMed.
- Z. Zhu, T. Chen, H.-F. Pi, H.-L. Ruan, J.-Z. Wu and P. Zhang, Chem. Nat. Compd., 2015, 51, 890–893 CrossRef CAS.
- Y.-y. Geng, Y.-j. Huang, J.-m. Wang, J. Zhou and Y. Hua, Zhongcaoyao, 2015, 46, 1111–1116 CAS.
- D.-L. Wang, A. Sowemimo, Y.-C. Gu, S. Gao, H.-B. Liu and P. Proksch, Fitoterapia, 2015, 105, 89–92 CrossRef CAS PubMed.
- T. Ohta, S. Nakamura, S. Nakashima, T. Matsumoto, K. Ogawa, K. Fujimoto, M. Fukaya, M. Yoshikawa and H. Matsuda, Tetrahedron, 2015, 71, 846–851 CrossRef CAS.
- V. K. Thu, N. Van Thang, N. X. Nhiem, B. H. Tai, N. H. Nam, P. V. Kiem, C. V. Minh, H. L. T. Anh, N. Kim, S. Park and S. H. Kim, Phytochemistry, 2015, 116, 213–220 CrossRef CAS PubMed.
- V. K. Thu, N. V. Thanga, N. X. Nhiem, H. L. T. Anh, P. H. Yen, C. V. Minh, P. V. Kiem, N. Y. Kim, S. J. Park and S. H. Kim, Nat. Prod. Commun., 2015, 10, 875–876 CrossRef PubMed.
- N.-L. Song, Z.-J. Li, J.-C. Chen, Y.-Y. Deng, M.-Y. Yu, L. Zhou and M.-H. Qiu, Phytochem. Lett., 2015, 13, 103–107 CrossRef CAS.
- W. Gao, C. Chen, J. Zhang, L. Cheng and D.-Y. Kong, Helv. Chim. Acta, 2015, 98, 60–66 CrossRef CAS.
- O. P. Noté, D. Jihu, C. Antheaume, M. Zeniou, D. E. Pegnyemb, D. Guillaume, H. Chneiwess, M. C. Kilhoffer and A. Lobstein, Carbohydr. Res., 2015, 404, 26–33 CrossRef PubMed.
- F. D. Mabou, D. Ngnokam, D. Harakat and L. Voutquenne-nazabadioko, Phytochem. Lett., 2015, 14, 159–164 CrossRef CAS.
- Y.-M. Won, Z.-K. Seong, J.-L. Kim, H.-S. Kim, H.-H. Song, D.-Y. Kim, J.-H. Kim, S.-R. Oh, H.-W. Cho, J.-H. Cho and H.-K. Lee, Arch. Pharmacal Res., 2015, 38, 1541–1551 CrossRef CAS PubMed.
- A. Alabdul Magid, H. Morjani, D. Harakat, C. Madoulet, V. Dumontet and C. Lavaud, Phytochemistry, 2015, 109, 49–56 CrossRef CAS PubMed.
- J. Zong, R. Wang, G. Bao, T. Ling, L. Zhang, X. Zhang and R. Hou, Fitoterapia, 2015, 104, 7–13 CrossRef CAS PubMed.
- X. Li, J. Zhao, X. Li, Y. Liu, Q. Xu, I. A. Khan and S. Yang, Helv. Chim. Acta, 2015, 98, 496–508 CrossRef CAS.
- P. Yang, X. Li, Y.-L. Liu, Q.-M. Xu, Y.-Q. Li and S.-L. Yang, J. Asian Nat. Prod. Res., 2015, 17, 800–807 CrossRef CAS PubMed.
- J. Wu, J. Zhao, Y. Liu, X. Li, Q. Xu, Y. Feng, I. A. Khan and S. Yang, Phytochem. Lett., 2015, 13, 379–385 CrossRef CAS.
- T. Morikawa, K. Ninomiya, Y. Takamori, E. Nishida, M. Yasue, T. Hayakawa, O. Muraoka, X. Li, S. Nakamura, M. Yoshikawa and H. Matsuda, Phytochemistry, 2015, 116, 203–212 CrossRef CAS PubMed.
- W. A. Elmasri, M.-E. F. Hegazy, Y. Mechref and P. W. Pare, RSC Adv., 2015, 5, 27126–27133 RSC.
- Z. Fan, L. Zhou, T. Xiong, J. Zhou, Q. Li, Q. Tan, Z. Zhao and J. Jin, Fitoterapia, 2015, 101, 19–26 CrossRef CAS PubMed.
- S. C. Heredia-Vieira, A. M. Simonet, W. Vilegas and F. A. Macías, J. Nat. Prod., 2015, 78, 77–84 CrossRef CAS PubMed.
- T. P. Nguyen, L. T. V. Hoa, M. D. Tri, L. T. Dung, P. N. Minh and B. T. Dat, Phytochem. Lett., 2015, 11, 102–105 CrossRef CAS.
- Y. Wang, L.-L. Zhang, C.-L. Zhang, Y.-F. Liu, D. Liang, H. Luo, Z.-Y. Hao, R.-Y. Chen and D.-Q. Yu, Phytochem. Lett., 2015, 11, 95–101 CrossRef CAS.
- H. Zha, Z. Wang, X. Yang, D. Jin, L. Hu, W. Zheng, L. Xu and S. Yang, Phytochem. Lett., 2015, 13, 108–113 CrossRef CAS.
- X.-R. Lu, X.-M. Wang, Z.-M. Wang, X.-Q. Chen, M.-Y. Wang and M.-X. Gong, Helv. Chim. Acta, 2015, 98, 245–252 CrossRef CAS.
- Q. Yu, J. Qi, L. Wang, S.-J. Liu and B.-Y. Yu, Phytother. Res., 2015, 29, 73–79 CrossRef CAS PubMed.
- C. Lavaud, C. Sayagh, F. Humbert, I. Pouny and C. Delaude, Carbohydr. Res., 2015, 402, 225–231 CrossRef CAS PubMed.
- D. Pertuit, T. Baghery Lotfabad, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2015, 98, 611–617 CrossRef CAS.
- H. Li, X.-Y. Wang, X.-Y. Wang, D. Hua, Y. Liu and H.-F. Tang, J. Asian Nat. Prod. Res., 2015, 17, 576–585 CrossRef CAS PubMed.
- M. Mohamed, O. Sterner and K.-E. Bergquistt, Life Sci. J., 2015, 12, 194–201 Search PubMed.
- G.-P. Zhou, Y. Yu, M.-M. Yuan, T. Ji, H.-Z. Fu and R.-J. Zhong, Molecules, 2015, 20, 9071–9083 CrossRef CAS PubMed.
- H.-Y. Kim, H. M. Kim, B. Ryu, J.-S. Lee, J.-H. Choi and D. S. Jang, Arch. Pharmacal Res., 2015, 38, 1963–1969 CrossRef CAS PubMed.
- H. Xiong, Y. Zheng, G. Yang, H. Wang and Z. Mei, Fitoterapia, 2015, 103, 33–45 CrossRef CAS PubMed.
- S. Aslan Erdem, A.-C. Mitaine-Offer, T. Miyamoto, M. Kartal and M.-A. Lacaille-Dubois, Phytochemistry, 2015, 110, 160–165 CrossRef CAS PubMed.
- R. C. Duarte, C. R. R. Matos, R. Braz-Filho and L. Mathias, Nat. Prod. Commun., 2015, 10, 871–874 CrossRef.
- I. Horo, M. Masullo, A. Falco, S. G. Şenol, S. Piacente and Ö. Alankuş-Çalişkan, Phytochem. Lett., 2015, 14, 39–44 CrossRef CAS.
- N. Windayani, L. D. Juliawaty, E. H. Hakim, K. Ruslan and Y. M. Syah, Nat. Prod. J., 2015, 5, 152–157 CAS.
- R. A. El Dib, J. Eskander, M. A. Mohamed and N. M. Mohammed, Fitoterapia, 2015, 106, 272–279 CrossRef CAS PubMed.
- Y.-y. Geng, Y.-j. Huang, J.-m. Wang, J. Zhou and Y. Hua, Tianran Chanwu Yanjiu Yu Kaifa, 2015, 27, 1134–1139 CAS.
- H.-Y. Chen, J.-H. Guh, S.-H. Chan and S.-S. Lee, Phytochem. Lett., 2015, 11, 229–235 CrossRef CAS.
- J. Hu, Y. Song, H. Li, B. Yang, X. Mao, Y. Zhao and X. Shi, Arch. Pharmacal Res., 2015, 38, 984–990 CrossRef CAS PubMed.
- Y. Lu, D. Van, L. Deibert, G. Bishop and J. Balsevich, Phytochemistry, 2015, 113, 108–120 CrossRef CAS PubMed.
- A. J. Pérez, A. M. Simonet, Ł. Pecio, M. Kowalczyk, J. M. Calle, F. A. Macías, W. Oleszek and A. Stochmal, Phytochem. Lett., 2015, 13, 165–170 CrossRef.
- S. C. V. A. R. Annam, A. Madhu, P. Mangala Gowri, T. V. Raju and B. V. V. Pardhasaradhi, Phytochem. Lett., 2015, 13, 370–374 CrossRef CAS.
- M. G. Ponnapalli, S. Sukki, S. C. H. V. A. R. Annam, M. Ankireddy, H. Tirunagari, V. R. Tuniki and V. V. P. Bobbili, Tetrahedron Lett., 2015, 56, 1570–1574 CrossRef CAS.
- B. Song, Q.-b. Zhang, M.-h. Wang, X.-h. Tian, H.-l. Sun, F.-b. Zhang, Z.-m. Zou and G. Ding, Zhongguo Zhongyao Zazhi, 2015, 40, 2144–2147 CAS.
- S. Cretton, L. Breant, L. Pourrez, C. Ambuehl, R. Perozzo, L. Marcourt, M. Kaiser, M. Cuendet and P. Christen, Fitoterapia, 2015, 105, 55–60 CrossRef CAS PubMed.
- J.-H. Han, W. Zhou, W. Li, P. Q. Tuan, N. M. Khoi, P. T. Thuong, M. K. Na and C.-S. Myung, J. Nat. Prod., 2015, 78, 1005–1014 CrossRef CAS PubMed.
- H.-F. Li, X.-A. Wang, S.-S. Xiang, Y.-P. Hu, L. Jiang, Y.-J. Shu, M.-L. Li, X.-S. Wu, F. Zhang, Y.-Y. Ye, H. Weng, R.-F. Bao, Y. Cao, W. Lu, Q. Dong and Y.-B. Liu, Drug Des., Dev. Ther., 2015, 9, 3017–3030 CrossRef CAS PubMed.
- Y. Ning, J. Huang, B. Kalionis, Q. Bian, J. Dong, J. Wu, X. Tai, S. Xia and Z. Shen, Stem Cells Int., 2015, 672312, DOI:10.1155/2015/672312.
- H. Dang, B. Feng, P.-y. Wang and X.-j. Wang, Zhongguo Shiyan Fangjixue Zazhi, 2015, 21, 216–221 CAS.
- P. Garg and A. Deep, Hygeia, 2015, 7, 18–27 CAS.
- J. M. R. Patlolla and C. V. Rao, Curr. Pharmacol. Rep., 2015, 1, 170–178 CrossRef CAS.
- Y.-Y. Guan, H.-J. Liu, X. Luan, J.-R. Xu, Q. Lu, Y.-R. Liu, Y.-G. Gao, M. Zhao, H.-Z. Chen and C. Fang, Phytomedicine, 2015, 22, 103–110 CrossRef CAS PubMed.
- M. Bahmani, A. Sarrafchi, H. Shirzad, N. Shahinfard, M. Rafieian-Kopaei, S. Shahsavari, B. Baharvand-Ahmadi, M. Taherikalani and S. Ghafourian, J. Chem. Pharm. Sci., 2015, 8, 683–692 CAS.
- L. Wang, R. Yang, B. Yuan, Y. Liu and C. Liu, Acta Pharm. Sin. B, 2015, 5, 310–315 CrossRef PubMed.
- A. Farozi, J. A. Banday and S. A. Shah, Beilstein J. Org. Chem., 2015, 11, 2707–2712 CrossRef CAS PubMed.
- J.-R. Yue, D.-Q. Feng and Y.-K. Xu, Nat. Prod. Res., 2015, 29, 1228–1234 CrossRef CAS PubMed.
- Y.-M. Pan, T. Zou, Y.-J. Chen, J.-Y. Chen, X. Ding, Y. Zhang, X.-J. Hao and H.-P. He, Phytochem. Lett., 2015, 12, 273–276 CrossRef CAS.
- T. Maffo, P. Wafo, R. S. T. Kamdem, R. Melong, P. F. Uzor, P. Mkounga, Z. Ali and B. T. Ngadjui, Phytochem. Lett., 2015, 12, 328–331 CrossRef CAS.
- B.-K. Xiao, J.-Y. Yang, Y.-R. Liu, J.-X. Dong and R.-Q. Huang, Chem. Nat. Compd., 2015, 51, 1126–1129 CrossRef CAS.
- L. Wang, P. Cao, Z. Zang, Y.-T. Ma, F.-Y. Liu, F. Li, X.-H. Wu, S.-X. Huang and Y. Zhao, Phytochem. Lett., 2015, 12, 168–172 CrossRef CAS.
- X. N. Nguyen, H. T. Yen, B. T. T. Luyen, B. H. Tai, V. H. Pham, H. L. T. Anh, N. K. Ban, V. K. Phan, V. M. Chau, J. H. Kim, J. M. Ni and Y. H. Kim, Bull. Korean Chem. Soc., 2015, 36, 703–706 CAS.
- Y. He, Y. Sun, D. Chen and P. Liu, Chem. Nat. Compd., 2015, 51, 273–275 CrossRef CAS.
- W.-H. Chen, C.-R. Han, Y. Hui, D.-S. Zhang, X.-M. Song, G.-Y. Chen and X.-P. Song, Helv. Chim. Acta, 2015, 98, 724–730 CrossRef CAS.
- J. Zhang, W. Wang, L. Qian, Q. Zhang, D. Lai and C. Qi, Oncol. Rep., 2015, 34, 2375–2384 CrossRef CAS PubMed.
- E.-S. Kim and A. Moon, Oncol. Lett., 2015, 9, 897–902 CrossRef PubMed.
- Y. Meng, Z.-M. Lin, N. Ge, D.-L. Zhang, J. Huang and F. Kong, Am. J. Chin. Med., 2015, 43, 1471–1486 CrossRef CAS PubMed.
- M. M. Farimani, M. B. Bahadori, S. A. Koulaei, P. Salehi, S. N. Ebrahimi, H. R. Khavasi and M. Hamburger, Fitoterapia, 2015, 106, 1–6 CrossRef CAS PubMed.
- M. M. Farimani, M. Abbas-Mohammadi, M.-A. Esmaeili, P. Salehi, S. Nejad-Ebrahimi, A. Sonboli and M. Hamburger, Planta Med., 2015, 81, 1290–1295 CrossRef CAS PubMed.
- A. M. F. Al-Aboudi, M. H. Abu Zarga, B. E. Abu-Irmaileh, F. F. Awwadi and M. A. Khanfar, Nat. Prod. Res., 2015, 29, 102–108 CrossRef CAS PubMed.
- S.-F. Tan, H.-J. Zhao, J.-G. Luo and L.-Y. Kong, Phytochem. Lett., 2015, 12, 1–5 CrossRef CAS.
- Y. Wu, J. Lu, X. Lu, R. Li, J. Guo, F. Guo and Y. Li, Phytochem. Lett., 2015, 13, 30–34 CrossRef CAS.
- H.-R. Wu, X.-F. He, X.-J. Jin, H. Pan, Z.-N. Shi, D.-D. Xu, X.-J. Yao and Y. Zhu, Fitoterapia, 2015, 106, 175–183 CrossRef CAS PubMed.
- J. P. Longue Ekon, A. N. Bissoue, M. Fomani, F. A. A. Toze, A. F. Waffo Kamdem, N. Sewald and J. D. Wansi, Z. Naturforsch., B: J. Chem. Sci., 2015, 70, 837–842 CAS.
- J.-H. Ma, Q.-H. Jiang, Y. Chen, X.-F. Nie, T. Yao, L.-Q. Ding, F. Zhao, L.-X. Chen and Q. Feng, Nat. Prod. Commun., 2015, 10, 2041–2044 Search PubMed.
- F.-M. Qin, B.-L. Liu, Y. Zhang and G.-X. Zhou, Nat. Prod. Res., 2015, 29, 633–637 CrossRef CAS PubMed.
- J.-l. Wang, D. Wang, J. Li, M. Zhao and S.-j. Zhang, Zhongcaoyao, 2015, 46, 3304–3309 CAS.
- C.-Q. Wang, M.-M. Li, W. Zhang, L. Wang, C.-L. Fan, R.-B. Feng, X.-Q. Zhang and W.-C. Ye, Fitoterapia, 2015, 106, 141–146 CrossRef CAS PubMed.
- K. O. Eyong, H. S. Foyet, G. Baïrys, G. Ngosong Folefoc, E. Acha Asongalem, A. Lagojda and M. Lamshöft, J. Ethnopharmacol., 2015, 174, 277–286 CrossRef CAS PubMed.
- L. Ali, A. L. Khan, L. Al-Kharusi, J. Hussain and A. Al-Harrasi, Mar. Drugs, 2015, 13, 4344–4356 CrossRef CAS PubMed.
- H.-Y. Wang, K. Liu, R.-X. Wang, S.-H. Qin, F.-L. Wang and J.-Y. Sun, Nat. Prod. Res., 2015, 29, 644–649 CrossRef CAS PubMed.
- C. Festa, M. V. D'Auria, V. Sepe, J. Ilaš, A. Leick, S. N'Gom and S. De Marino, Phytochem. Lett., 2015, 13, 324–329 CrossRef CAS.
- J. R. Chouna, J.-d.-D. Tamokou, P. Nkeng-Efouet-Alango, B. N. Lenta and N. Sewald, Z. Naturforsch., C: J. Biosci., 2015, 70, 169–173 CAS.
- X.-W. Zhao, J.-D. Zhong, H.-M. Li and R.-T. Li, Phytochem. Lett., 2015, 12, 308–312 CrossRef CAS.
- P. Wu, H. Gao, Z. H. Li and Z. Q. Liu, Phytochem. Lett., 2015, 12, 17–21 CrossRef CAS.
- C. Baecker, K. Jenett-Siems, K. Siems, T. H. J. Niedermeyer, M. Wurster, A. Bodtke and U. Lindequist, Z. Naturforsch., B: J. Chem. Sci., 2015, 70, 403–408 CAS.
- S. Li, J. Zhao, W. Wang, Y. Lu, Q. Xu, Y. Liu, X. Li, I. A. Khan and S. Yang, Phytochem. Lett., 2015, 14, 178–184 CrossRef CAS.
- B. Yang, T. Yang, Q. Tan, J. Zhu, L. Zhou, T. Xiong, Z. Zhao and J. Jin, Phytochem. Lett., 2015, 13, 302–307 CrossRef CAS.
- D. Chauhan and J. Singh, World J. Pharm. Res., 2015, 4, 2719–2723 CAS.
- S.-J. Xiao, F. Chen, L.-S. Ding and Y. Zhou, Chin. J. Nat. Med., 2015, 13, 65–68 CAS.
- K. W. Woo, S. U. Choi, K. H. Kim and K. R. Lee, J. Braz. Chem. Soc., 2015, 26, 1450–1456 CAS.
- S. Y. Lee, W. S. Suh, H. K. Kim, I. K. Lee, S. U. Choi, K. H. Kim and K. R. Lee, Heterocycles, 2015, 91, 1187–1197 CrossRef CAS.
- S.-G. Li, M.-M. Li, B.-X. Zhao, Y. Wang and W.-C. Ye, J. Asian Nat. Prod. Res., 2015, 17, 1153–1159 CrossRef CAS PubMed.
- L. Ali, R. Ahmad, N. Ur Rehman, A. Latif Khan, Z. Hassan, T. Shamim Rizvi, A. Al-Harrasi, Z. Khan Shinwari and J. Hussain, Helv. Chim. Acta, 2015, 98, 1240–1244 CrossRef CAS.
- W. Li, L. Y. Li, W. Zhou, I. Hwang, J. Y. Ma and Y. H. Kim, Arch. Pharmacal Res., 2015, 38, 2124–2130 CrossRef CAS PubMed.
- B. K. Chhetri, N. S. Dosoky and W. N. Setzer, Planta Med. Lett., 2015, 2, e73–e77 CrossRef.
- L.-Y. Zhang, T.-H. Wang, L.-Z. Ren, M.-Z. Wan, H.-F. Wu, Q.-X. Mei and Y.-H. Gao, Biochem. Syst. Ecol., 2015, 59, 155–158 CrossRef CAS.
- V. T. Nguyen, D. C. To, M. H. Tran, S. H. Oh, J. A. Kim, M. Y. Ali, M.-H. Woo, J. S. Choi and B. S. Min, Bioorg. Med. Chem., 2015, 23, 3126–3134 CrossRef CAS PubMed.
- E. C. Marfori, S. I. Kajiyama, E.-i. Fukusaki and A. Kobayashi, J. Pharmacogn. Phytochem., 2015, 3, 140–143 CAS.
- P. S. Achanta, R. K. Gattu, A. R. V. Belvotagi, R. R. Akkinepally, R. K. Bobbala and A. R. V. N. Achanta, Fitoterapia, 2015, 100, 166–173 CrossRef CAS PubMed.
- F. Messina, M. Curini, C. Di Sano, C. Zadra, G. Gigliarelli, L. A. Rascón-Valenzuela, R. E. Robles Zepeda and M. C. Marcotullio, J. Nat. Prod., 2015, 78, 1184–1188 CrossRef CAS PubMed.
- Y. Li, H. Tang, X. Tian, H. Lin, M. Wang and M. Yao, Fitoterapia, 2015, 106, 226–230 CrossRef CAS PubMed.
- C.-L. Zhang, Y. Wang, Y.-F. Liu, D. Liang, Z.-Y. Hao, H. Luo, Q.-J. Zhang, G.-R. Shi, R.-Y. Chen, Z.-Y. Cao and D.-Q. Yu, Tetrahedron, 2015, 71, 5579–5583 CrossRef CAS.
- C.-L. Zhang, Y.-F. Liu, Y. Wang, D. Liang, Z.-B. Jiang, L. Li, Z.-Y. Hao, H. Luo, G.-R. Shi, R.-Y. Chen, Z.-Y. Cao and D.-Q. Yu, Org. Lett., 2015, 17, 5686–5689 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |