Study of “Ni-doping” and “open-pore microstructure” as physico-electrochemical stimuli towards the electrocatalytic efficiency of Ni/NiO for the oxygen evolution reaction†
Abstract
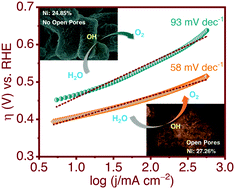
In the quest to augment the performance of electrocatalysts for application in ultra-efficient energy conversion and storage devices based on oxygen electrochemistry, in this work, we have demonstrated a facile procedure to simultaneously modulate the amount of Ni-doping and the pore architecture of NiO, to synthesize Ni/NiO with largely improved electrocatalytic efficiency towards the oxygen evolution reaction (OER) in alkaline medium. Results show that additional amalgamation of Ni along with the open-pore architecture of Ni/NiO significantly improves the conductivity and offers additional active sites, which in combination, facilitate the charge transfer, lower the onset OER potential, improve the reaction kinetics and increase the overall turnover frequency during the electrocatalytic OER. The chemical and microstructural modification of Ni/NiO also stimulates the poisoning-resistant behaviour and electromechanical stability of the material, during the electrocatalytic OER for longer duration. Significant current and potential oscillations are observed in the “i vs. t” and “V vs. t” plots, from where a major mechanistic conclusion is drawn regarding the very fast OER kinetics and the poisoning-resilient behaviour of Ni/NiO against (–OH and –O)ads, during the electrocatalytic OER. The presented approach can be a reference for the future design of all non-noble metal OER electrocatalysts, towards application in ultra-efficient energy conversion and storage devices based on oxygen electrochemistry.


 Please wait while we load your content...
Please wait while we load your content...