A supramolecular adduct of tegafur and syringic acid: the first tegafur-nutraceutical cocrystal with perfected in vitro and in vivo characteristics as well as synergized anticancer activities†
Abstract
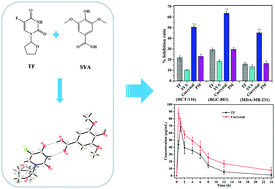
With the objectives of ameliorating the in vitro biopharmaceutical properties and optimizing in vivo pharmacokinetic characteristics as well as enhancing anticancer activities of tegafur (TF), and thereby acquiring some new insights into the development of a synergistic antitumor pharmaceutical cocrystal, a supramolecular adduct of TF and syringic acid (SYA), namely TF–SYA, is obtained as the first tegafur-nutraceutical cocrystal. Its accurate structure determined by single-crystal X-ray diffraction shows that both TF and SYA in the crystalline lattice have a stoichiometric ratio of 1 : 1, in which the two components contain a cyclic hydrogen-bonding couple, and then the couples are assembled to a supramolecular hydrogen-bonding bilayer containing offsetting parallel π–π interactions. The dissolving and permeating behaviors are investigated under various physiological pH environments to evaluate the effects of cocrystallization on the physicochemical properties of the cocrystal. The conclusion that the water solubility and permeability of TF from the TF–SYA cocrystal are concurrently enhanced on comparing with intact TF can be drawn. Intriguingly, the ameliorated in vitro biopharmaceutical properties can efficaciously turn into the in vivo optimized pharmacokinetic characteristics with the prolonged half-life and increased bioavailability compared with TF itself. Even more noteworthy is that both TF and SYA in the cocrystal can give rise to increasing cytotoxicity, showing synergistic antitumor effects against the tested cell lines. Thus, the contribution of this study not only provides a new solid-state crystalline form for TF with promising application prospects, but also explores a new pathway for the development of synergistic antitumor cocrystal drugs based on a cocrystallization strategy.


 Please wait while we load your content...
Please wait while we load your content...