Novel phenyl and thiophene dispiro indenoquinoxaline pyrrolidine quinolones induced apoptosis via G1/S and G2/M phase cell cycle arrest in MCF-7 cells†
Abstract
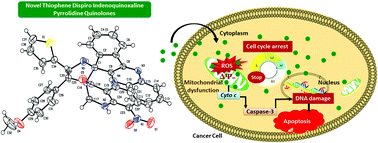
New phenyl and thiophene dispiro indeno quinoxaline pyrrolidine quinolone analogues were synthesized by a one-pot four-component [3+2] cycloaddition reaction between (E)-3-arylidene-2,3-dihydro-8-nitro-4-quinolones, o-phenylenediamine, ninhydrin, and benzylamine/thiophenemethylamine. The structure and regio- and stereochemistry of the compounds have been confirmed by various spectroscopic and X-ray diffraction analyses. The observed regio- and stereoselectivity of the compounds were further enlightened by DFT calculations. Further, the in vitro cytotoxic effects of the compounds were evaluated by MTT assay. The compounds exhibited significant inhibition activity towards the tested cancer cells HeLa and MCF-7. Among the synthesized compounds compound 9e showed the highest inhibitory potency against MCF-7 cells. Mechanistic cell death studies revealed that cell death was induced by 9e against MCF-7 cells by ROS mediated G1/S and G2/M phase cell cycle arrest apoptosis. The apoptotic cell death was quantified by the annexin V-FITC/PI staining method. The binding mode of compound 9e was further examined by in silico molecular docking studies.


 Please wait while we load your content...
Please wait while we load your content...