Improved photoremoval performance of boron carbon nitride–pyromellitic dianhydride composite toward tetracycline and Cr(vi) by itself to change the solution pH†
Abstract
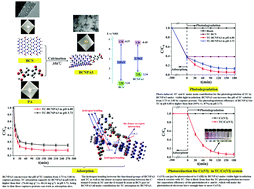
A series of boron carbon nitride–pyromellitic dianhydride (BCNPA) composites were successfully synthesized for the first time, where BCNPA3 exhibited the best adsorption and photodegradation performances for tetracycline (TC) under visible-light irradiation. 1H NMR characterization confirmed the fact that BCNPA3 was formed via the dehydration reaction between the OH groups in BCN and COOH groups in PA. Elemental N in BCNPA3 could capture protons and increase the pH of the TC solution from 3.73 to 6.80; consequently, TC changed from the positive state (pH 3.73) to the neutral state (pH 6.80). Subsequently, the adsorption capacity of BCNPA3 for TC increased from 66.24 mg g−1 (at pH 3.73) to 74.66 mg g−1 (at pH 6.80). This may be attributed to the fact that these captured protons can work as adsorption sites, too. Furthermore, the photodegradation efficiency of BCNPA3 for TC at pH 6.80 under visible-light irradiation was also higher than that at pH 3.73 (95% vs. 87%), because TC at pH 6.80 is less stable than that at pH 3.73. More interestingly, Cr(VI) could only be photoreduced into Cr(III) by BCNPA3 under visible-light irradiation when it coexisted with TC. This could be attributed to the fact that the photodegradation of TC increased the separation efficiency of the photoinduced electron and hole; consequently, the photoinduced electrons had sufficient time to reduce Cr(VI). Photoinduced ˙O2− and holes had the main contribution toward TC photodegradation by BCNPA3, while photoinduced electrons were responsible for Cr(VI) photoreduction. The adsorption and photodegradation performances of BCNPA3 for TC and Cr(VI) could be easily recovered under visible-light irradiation.


 Please wait while we load your content...
Please wait while we load your content...