Binding of Fe(ii)-complex of phenanthroline appended glycoconjugate with DNA, plasmid and an agglutinin protein†
Abstract
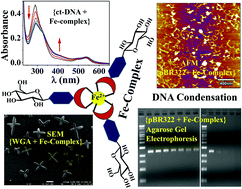
The phenanthroline appended glycoconjugate (L) and its Fe(II)-complex were synthesized and characterized using analytical and spectral methods. The complexation of Fe2+ with L has been shown using absorption and fluorescence spectral studies. 1H NMR titration of L with Fe2+ supports that the phenanthroline moiety acts as the metal binding core. The interaction and binding of the Fe-complex with calf thymus DNA (ct-DNA) were studied using spectral techniques, such as UV-Vis absorption, circular dichroism (CD) and DNA melting temperatures. The corresponding data supported the minor groove binding of ct-DNA by the Fe-complex along with electrostatic interactions. The gel electrophoresis studies carried out using pBR322 plasmid showed a tight binding of the iron complex with the plasmid and this resulted in the condensation of the pBR322 plasmid as revealed by the blob-like structures observed in the atomic force microscopy data. The binding of the Fe-complex by WGA was demonstrated by absorption, fluorescence and CD spectra, and the microstructures were observed via scanning electron microscopy (SEM). Star-like nanostructures observed via SEM support that the octahedral iron complex connects the protein molecules in three dimensions through their carbohydrate terminal moieties. Thus, a new Fe-complex possessing a biocompatible glucosyl moiety interacts and binds to biomolecules, such as DNA and protein, and the resulting species are of importance in biological applications.


 Please wait while we load your content...
Please wait while we load your content...