Open Access Article
Open Access ArticleNMR probe effects on trans-philicity and trans-influence ladders in square planar Pt(II) complexes†
Athanassios C.
Tsipis

Department of Chemistry, University of Ioannina, Ioannina 45110, Greece. E-mail: attsipis@uoi.gr
First published on 27th April 2020
Abstract
Quantitative trans-philicity ladders for a broad series of ligands in square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes are built employing the isotropic σiso(SO) X NMR shielding constants, calculated by DFT computational protocols at the SO-ZORA level of theory, as the trans-philicity descriptors. Linear relationships between the σiso(SO) X trans-philicity descriptors and the R(Pt–X) descriptors of trans-influence demonstrate the relation of trans-philicity with trans-influence. The electronic features of the probes are crucial factors that manipulate trans-philicity. The isotropic σiso(SO) X NMR descriptors of trans-philicity linearly correlated with the ligand electronic PL constants and other popular electronic/structural descriptors related with the L–Pt–X bonding, revealed the origin of trans philicity. The trans-philicity ladders constructed by the six different probes go roughly parallel with only minor deviations related with the position of L in the rungs of the ladders.
Introduction
There have been numerous studies concerning the effect of a ligand on the lability of other ligands in transition metal complexes. The great majority of these investigations have been concerned with the trans-effect in square planar metal complexes and has been shown to be more important than the cis-effect which is thought to be very small and difficult to predict.1–8 In parallel with the trans-effect term Pidcock et al.9 introduced the trans-influence term. Across the periodic table the trans-influence operates, whereby tightly bonded ligands selectively lengthen mutually trans metal–ligand bonds. The trans-influence is fundamentally important and underpins the trans-effect, a kinetic rate effect where the order of substitution of ligands at a metal centre can be controlled.Recently we aimed to gain a comprehensive understanding of the trans-effect/trans-influence phenomena for a broad series of octahedral [Cr(CO)5L]−/0/+ complexes employing the calculated σiso 13C NMR shielding constants as the trans-effect/trans-influence metrics introducing the concept of trans-philicity to cover both kinetic and equilibrium phenomena.10trans-Philicity combines two discriminate electronic effects responsible for the electron density transfer either through the σ- or the σ- and π-subspaces. In this context the strength of trans-philicity could be explained in terms of σ-donation and π-back-donation, both being electronic effects. These electronic effects have previously been quantified by well-established ligand electronic parameters, such as the PL constants defined as PL = E1/2[Cr(CO)6] − E1/2[Cr(CO)5L].11–13
In a following paper14 we applied the trans-philicity concept in the realm of square planar Pt(II) complexes where both trans-influence and trans-effects have frequently been epitomized and probe whether and to what extent the cis ligands affect trans philicity. Having in mind that trans-effect/trans-influence phenomena operate mutually along a linear L–M–X framework we report herein on the effect of the leaving group X (used as a NMR probe) on the trans-philicity and trans-influence ladders for a broad series of square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes involving a wide variety of L (44 ligands) with diverse electronic features (σ-donor, σ-donor/π-donor, σ-donor/π-acceptor ligands). The trans-philicity and trans-influence ladders are built employing the calculated σiso X NMR shielding constants and the R(Pt–X) bond lengths respectively. Linear correlations between NMR parameters and the well established ligand electronic parameter PL and other popular electronic/structural descriptors related with the L–Pt–X bonding threw light on the underlying principles and the origin of trans philicity and validates the broad relevance across inorganic and organometallic chemistry and catalysis, disposing a powerful tool in the arsenal of modelling and designing techniques.
Computational methods
All calculations were performed using the Gaussian 09, version D.01 program suite.15 The geometries of the complexes were fully optimized, without symmetry constraints, employing the 1999 hybrid functional of Perdew, Burke, and Ernzerhof16–19 as implemented in the Gaussian09, version D.01 program suite. The PBE0 functional mixes the Perdew–Burke–Ernzerhof (PBE) exchange energy and Hartree–Fock exchange energy in a set 3 to 1 ratio, along with the full PBE correlation energy. Geometry optimization of the square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes was done in solution (benzene solvent) using the all electron SARC_ZORA basis set20,21 for Pt central atom and the 6-31+G(d) basis set for all main group elements (E). Solvent effects were accounted for by means of the Polarizable Continuum Model (PCM) using the integral equation formalism variant (IEF-PCM) being the default self-consistent reaction field (SCRF) method.22 Hereafter the computational protocol used in DFT calculations is abbreviated as PBE0/SARC-ZORA(Pt)∪6-31+G(d)(E)/PCM. All stationary points have been identified as minima (number of imaginary frequencies NImag = 0). Natural Bond Orbital (NBO) population analysis was performed using Weinhold's methodology.23,24 Magnetic shielding tensors have been computed with the gauge-including atomic orbitals DFT method,25,26 as implemented in the Gaussian09 series of programs. The NMR shielding constants were calculated by inclusion of spin–orbit (SO) effects at the 2-component-spin–orbit-ZORA (SO-ZORA) level of theory,27,28 using the ADF2019 code.29 For simplicity we will denote the isotropic shielding constants as σiso(SO) calculated at the GIAO (SO-ZORA)/PBE0/TZ2P/COSMO level of theory. The GIAO (SO-ZORA)/PBE0/TZ2P/COSMO computational protocol was chosen for it was successfully used by Kaupp and co-workers30 to probe the trans influence on 1H NMR hydride shifts in square-planar platinum(II) complexes predicting accurate 1H NMR hydride shifts matching experimental data available. We also performed calculations of the 1H NMR hydride shifts for selected complexes employing the GIAO (SO-ZORA)/PBE0/TZ2P-J/COSMO and the results are given in the ESI,† (Table S1). It can be seen that both computational protocols provide 1H NMR hydride shifts comparable to 1H NMR hydride shifts calculated by Kaupp and co-workers30 and the experiment. The deviations observed are due to the fact that in our calculations the 1H NMR hydride shifts are calculated for the optimized geometries of the platinum(II) complexes in solution (benzene solvent), while the calculations performed by Kaupp and co-workers30 referred to the gas-phase optimized geometries. Notice that the estimated R(Pt–H) bond lengths for the optimized geometries in solution, as it was expected, are longer by 0.053 Å relative to the R(Pt–H) bond lengths of the optimized geometries in gas-phase (Table S2, ESI†). Obviously the estimated R(Pt–H) bond lengths accounts well for the observed deviations of the 1H NMR hydride shifts.Results and discussion
Selection of the NMR probes
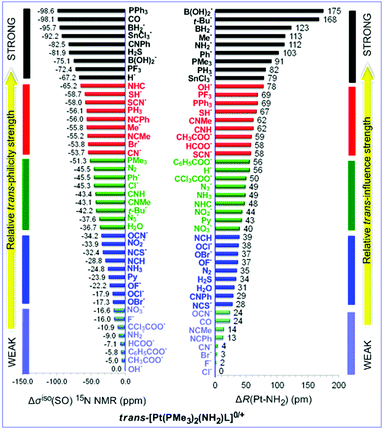
trans-Philicity ladders have been built for square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes for a broad series of ligands L (44 ligands) employing 1H, 13C, 15N, 17O and 35Cl NMR probes. We selected six different common NMR probes in order to answer the question whether the nature of the NMR probe affects the trans-philicity sequences (ladders) and the σisotrans-philicity descriptors. The selection of the NMR probes (X) was based on their electronic characteristics, e.g. employing the strong σ-donor H− and CH3− ligands, the σ-donor/π-acceptor CO ligand, the σ-donor/π-donor NH2− and Cl− ligands and the weak σ-donor/weak π-donor OH2 ligand. Moreover the selected probes are ligands of broad relevance across platinum chemistry. The H− and CH3− ligands are formed in catalytic processes involving activation and functionalization of C–H bonds or oxidative addition reactions.31–34 and dehydrocoupling reactions of compounds with element-hydrogen bonds.35 The Cl− ligand is a good leaving group for the anticancer cis-Pt(NH3)2Cl2 (cis-platin) drug, the OH2 ligand occurs in the hydrolysis products of cis-platin, while the NH2− ligand models the guanine base of DNA which is generally conceived to be a major bio-molecular target of the classic Pt(II) drugs.36–38trans-Influence ladders have also been constructed employing the R(Pt–X) structural parameters. The relative trans-philicity strengths are expressed by the Δσiso X NMR trans-philicity metrics defined as the difference between the calculated σiso X NMR shielding constants for the complete set of ligands and the σiso X NMR shielding constant of the complex containing the ligand exhibiting the weakest trans-philicity. Similarly the relative trans-influence strengths are expressed by the ΔR(Pt–X) metrics.trans-Philicity ladders constructed by the strong σ-donor H− and CH3− NMR probes
The trans-philicity ladders for the trans-Pt(PMe3)2(H)L and trans-[Pt(PMe3)2(CH3)L]0/+ complexes involving the 1H and 13C NMR probes quantified by the Δσiso(SO) 1H and 13C NMR metrics along with the trans-influence ladders quantified by the ΔR(Pt–H) and ΔR(Pt–CH3) metrics are shown in Chart 1. The calculated σiso(SO) 1H and 13C NMR shielding constants are given in the ESI† (Tables S3 and S4).Perusal of Chart 1 reveals that the 1H and 13C NMR trans-philicity ladders retrieve well the experimentally established trans orienting series:39
| H2O ≈ NO3− < OH− < NH3 < Cl− < Br− < I− ≈ SCN− ≈ NO2− ≈ PR3 ≪ CO ≈ C2H4 ≈ CN− ≈ CH3− ≈ H− |
Chval et al.39 thoroughly investigated the mechanism of anation reactions in square planar trans-Pt[(NH3)2T(H2O)]n+ complexes (T = H2O, NH3, OH−, F−, Cl−, Br−, H2S, CH3S−, SCN−, CN−, PH3, CO, CH3−, H−, C2H4) employing DFT computational methods. The authors showed that for trans ligands with a very strong σ-donation (e.g. CH3− and H−) the substitution proceeds by a dissociative interchange (Id) mechanism, for trans ligands with strong π-back donation (e.g. C2H4) the substitution proceeds by a two step associative mechanism and for trans ligands with weak σ-donation and π-back-donation the substitution reactions proceed by an associative interchange (Ia) mechanism. According to the computed activation energies the T ligands follow the trans effect sequence:
| C2H4 ≫ CH3− ≈ H− > CO ≈ CH3S− ≈ PH3 > CN− ≈ NO2− ≈ H2S > Br− > Cl− > SCN− ≈ NH3 ≈ OH− > F− ≈ H2O |
The calculated σiso(SO) shielding constants for selected trans-Pt(PMe3)2(H)L for which experimental data are available40–44 (cf. Table S1, ESI†) demonstrate that the calculated σiso(SO) shielding constants are accurate metrics to deploy the ligands L in reliable trans-philicity ladders (trans-philicity sequences).
Comparison of the 1H and 13C NMR trans-philicity ladders reveals that the two ladders are almost identical. In both ladders the strong σ-donors (H−, Me−, BH2−, B(OH)2− and SnCl3−) occupy the rungs with very strong trans-philicity (black rungs), the C-donor ligands the rungs with strong trans-philicity (red rungs) while the N- and O-donor ligands occupy the rungs with moderate to weak trans-philicity (green and blue rungs). However in many cases the ligands L follow different orders along the trans orienting series. In particular the strong σ-donors t-Bu− and Ph− ligands occupy remarkably different rungs in the 1H and 13C NMR trans-philicity ladders. The t-Bu− and Ph− ligands are found in the black rungs of the 1H NMR trans-philicity ladder and the green rungs of the 13C NMR trans-philicity. However this is not the case in the respective trans-influence sequences (ladders). It is important to be noticed that Kaupp and co-workers30 applying quantitative relativistic DFT methodology in a series of square planar Pt(II) complexes and exploring correlations between the calculated 1H shifts and the trans ligand influence series established the trans-influence sequence: NO3− < ONO− < NO2− < Cl− < Br− < SCN− ≈ I− < CN− < Ph− < Me− < SiR3− ≈ BR2−, which is exactly the same with the trans-philicity series shown in the σiso(SO) 1H NMR trans-philicity ladder (Chart 1). In a following publication Kaupp and co-workers45 presented the results of a extensive investigation of the ligand effects on the NMR shifts of metal-bound nuclei in 5d transition-metal complexes, encompassing both 5d8 and 5d10 electron configurations, with related effects even for 5d6 complexes using relativistic quantum-chemical analyses. The authors showed that the trans ligand effects on the shieldings are exclusively dominated by two mixed σ-/π-type spinors.
Generally with only minor deviations related with the position of a few ligands in the trans-influence and 1H NMR trans-philicity ladders the two ladders go parallel to each other. Phosphanes, nitriles, CO, NO2−, OH−, and N2 exert strong trans-influence (R(Pt–H) = 1625–1638 pm), while the O-donor ligands along with halides, isocyanides, SH2, N3−, NCS−, Py, NH3, N2 and OH2 ligands exert moderate to weak trans-influence (R(Pt–H) = 1584–1620 pm). Noteworthy in both 1H and 13C NMR trans-philicity ladders the trans-philicity of phosphane ligands follows the order: PF3 > PH3 > PPh3 > PMe3. According to the σ-donor/π-acceptor ratio, PF3, PH3 and PMe3 follow the trend46 PF3 < PH3 < PMe3, while according to the ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) stretching vibrational frequencies for Ni(CO)3L complexes follow the order47 PF3 > PPh3 > PMe3 in line with the trans-philicity sequence for the phosphane ligands. Similarly the 1H and 13C NMR trans-philicity ladders reproduce the experimentally established trans-influence sequences, Br− > Cl− > F− and NH3 > Py.
O) stretching vibrational frequencies for Ni(CO)3L complexes follow the order47 PF3 > PPh3 > PMe3 in line with the trans-philicity sequence for the phosphane ligands. Similarly the 1H and 13C NMR trans-philicity ladders reproduce the experimentally established trans-influence sequences, Br− > Cl− > F− and NH3 > Py.
In the trans-[Pt(PMe3)2(CH3)L]0/+ complexes the trans-influence ladder match better to the 13C NMR trans-philicity ladder. The two ladders are almost similar showing only minor local order inversions of a few ligands along the trans orienting series. In both ladders the strong σ-donor (H−, Me− and t-Bu−, SnCl3−), the C-donor (CO and isocyanides) along with the BH2−, B(OH)2− and phosphane ligands occupy the rungs with strong to very strong trans-influence and trans-philicity. Similarly the N- and O-donor ligands occupy the rungs with moderate to weak trans-influence and trans-philicity, but in many cases follow different orders along the trans orienting series.
trans-Philicity ladder constructed by the σ-donor/π-acceptor CO probe
The trans-philicity ladder for the trans-[Pt(PMe3)2(CO)L]+/2+ complexes quantified by the Δσiso 13C NMR metrics along with the trans-influence ladder quantified by the ΔR(Pt–CO) metrics are shown in Chart 2. The calculated σiso(SO) 13C NMR shielding constants are given in the ESI† (Table S5).The 13CO NMR trans-philicity ladder matches better to the 1H NMR than the 13CH3 NMR ladders (Chart 1). Comparison of the aforementioned trans-philicity ladders illustrates clearly that the electronic features of the probes are crucial factors that tune deploy of ligands L in the trans-philicity ladders. In particular nitriles NCR (R = H, Me, Ph) occupy the pale blue rungs with weak trans-philicity in the 13CO NMR trans-philicity ladder and the blue rungs with moderate trans-philicity in the 13CH3 and 1H NMR trans-philicity ladders. It can also be seen that various classes of ligands in the three ladders follow the trends:
Strong σ-donor ligands:
| 1H ladder: SnCl3− < t-Bu− < Ph− < Me− < H− < BH2− < B(OH)2− |
| 13CH3 ladder: t-Bu− < Ph− < Me− < SnCl3− < H− < B(OH)2− < BH2− |
| 13CO ladder: SnCl3− < Ph− < Me− < H− < t-Bu− < B(OH)2− < BH2− |
C-Donor ligands:
| 1H ladder: CNMe < NHC < CNH < CNPh < CN− < CO |
| 13CH3 ladder: CN− < NHC < CNPh < CNMe < CNH < CO |
| 13CO ladder: CO < CNMe < CNPh < NHC < CNH < CN− |
N-Donor ligands:
| 1H ladder: N2 < py < NCH < NCMe < NO2− < NH3 < NCPh < N3− < NCS− < NH2− |
| 13CH3 ladder: NO2− < NCS− < N3− < py ≪ NH2− < NCMe < NCPh < NH3 < NCH < N2 |
| 13CO ladder: N2 < NCH < NCMe < NCPh < NH3 < py < NCS− < NO2− < N3− < NH2− |
O-Donor ligands:
| 1H ladder: OH2 < OCN− < NO3− < CCl3COO− < OCl− < OBr− < HCOO− < CH3COO− < C6H5COO− < OF− < OH− |
| 13CH3 ladder: OCl− < OBr− < OF− < NO3− < OCN− < CH3COO− < C6H5COO− < OH− < HCOO− < CCl3COO− < OH2 |
| 13CO ladder: OH2 < OCN− < NO3− < CCl3COO− < HCOO− < CH3COO− < C6H5COO− < OCl− < OBr− < OF− < OH− |
Phosphanes:
| 1H ladder: PMe3 < PPh3 < PH3 < PF3 |
| 13CO ladder: PMe3 < PPh3 < PH3 < PF3 |
| 13CH3 ladder: PF3 < PH3 < PMe3 < PPh3 |
The deviations observed might be due to the synergic contribution of the σ-donor and π-acceptor capacity of the probes to the mutual electron density transfer L → Pt → CO pathways (channels) taken place through the σ- and/or the σ- and π-subspaces (Scheme 1).
 | ||
| Scheme 1 Electron density transfer pathways (σ- and π-channels) between the trans L ligands and the X NMR probes supported by σ- and π-MOs. | ||
According to Scheme 1 the electronic features of the trans L ligands and X NMR probes are the crucial determinants of the net charge transfer from the trans L ligands to X NMR probes that manipulates trans-philicity. In this context trans-philicity (trans effect/trans-influence) originates from electronic effects. The electronic nature of trans-philicity accounts well for the positions of the σ-donor/π-donor SH−, SCN−and NH2− ligands in rungs of higher trans-philicity relative to their positions in the 1H and 13CH3 NMR ladders. Coordination of the σ-donor/π-donor ligands to [Pt(PMe3)2(CO)]2+ reference standard adds more electron density on the CO probe by electron density transfer through the π-channel that increases the downfield shifts, hence increasing trans-philicity and moving the positions of σ-donor/π-donor ligands in rungs of higher trans-philicity in the 13CO NMR trans-philicity ladder.
Generally in the trans-influence ladder the majority of the ligands occupy the proper rungs of the ladder, e.g. the strong σ-donors and phosphanes occupy the rungs of very strong trans-influence, the C-donors the rungs of strong trans-influence, the N-donors, hypohalites, SH2 and Cl− the rungs of moderate trans-influence and the O-donors along with F− the rungs of weak trans-influence.
trans-Philicity ladder constructed by the strong σ-donor/π-donor NH2 probe
The trans-philicity ladder for the trans-[Pt(PMe3)2(NH2)L]0/+ complexes quantified by the Δσiso 15N NMR metrics along with the trans-influence ladder quantified by the ΔR(Pt–NH2) parameters are shown in Chart 3. The calculated σiso(SO) 15N NMR shielding constants are given in the ESI† (Table S6).In the 15N NMR trans-philicity ladder the O-donor ligands occupy the rungs with weak trans-philicity, the strong σ-donor and phosphane ligands the rungs with strong trans-philicity, while the N- and C-donor ligands occupy rungs with strong, moderate and weak trans-philicity. Noteworthy the 15N NMR trans-philicity ladder matches better to the 13CH3 ladder, with only minor deviations related with the position of L in the rungs of the two ladders, rather than to the 13CO and 1H NMR trans-philicity ladders. However in the trans-influence ladder remarkable changes in the trans-influence sequences relative to the 15N NMR trans-philicity sequences are observed. Specifically in the trans-influence ladder the O-donor ligands are placed in the rungs of moderate to strong trans-influence. In the trans-influence sequences phosphanes are placed in the rungs with weak to very weak trans-influence. The same holds true for some of the C-donor ligands (CO, CN- and isocyanides) and N-donor ligands (NHC, Py, NH3 and nitriles) deviating from the experimentally established trans orienting series.
trans-Philicity ladder constructed by the weak σ-donor/weak π-donor OH2 probe
The trans-philicity ladder for the trans-[Pt(PMe3)2(OH2)L]+/2+ complexes quantified by the Δσiso 17O NMR descriptors along with the trans-influence ladder quantified by the ΔR(Pt–OH2) structural parameters are shown in Chart 4. The calculated σiso(SO) 17O NMR shielding constants are given in the ESI† (Table S7).Interestingly the 17O NMR trans-philicity ladder is almost identical with the 1H NMR (Chart 1) and 13CO NMR (Chart 2) trans-philicity ladders with only marginal deviations related with local inversion of the trans-philicity order of some ligands. On the other hand the trans-influence ladder quantified by the R(Pt–OH2) parameters deploy trans-influence sequences, which do not match the experimentally established trans orienting series. In effect the strong σ donor BH2−, B(OH)2−, H−, SnCl3−, Me−, t-Bu− and Ph− anionic ligands along with phosphanes, CN−, NH2−, SH−, SCN− and NO2− ligands occupy the rungs with very strong trans-influence in the trans-influence ladder in line with the experimentally established trans orienting series. However the O-donor (RCOO−, OX−, OCN− and OH−) ligands along with NHC, N3−, Br−, Cl−, NCS− and PH3 occupy the rungs with strong to moderate trans-influence deviating from the experimentally established trans orienting series. Similarly the C-donor (isocyanides and CO) ligands along with the N-donor (nitriles, NH3, Py and N2) and PF3 ligands are placed in the rungs of weak trans-influence also deviating from the experimentally established trans orienting series.
trans-Philicity ladder constructed by the moderate σ-donor/weak π-donor Cl probe
Chart 5 shows the trans-philicity ladder for the trans-[Pt(PMe3)2(Cl)L]0/+ complexes quantified by the Δσiso(SO) 35Cl NMR descriptors along with the trans-influence ladder quantified by the ΔR(Pt–Cl) structural descriptors. The calculated σiso(SO) 35Cl NMR shielding constants are given in the ESI† (Table S8).The 35Cl NMR trans-philicity ladder has an analogous structure to the corresponding 1H, 13CO and 17O NMR trans-philicity ladders with only minor deviations related with the positions of a few ligands in the rungs of the ladders. Surprisingly the rungs with very strong trans-philicity are occupied by the C-donor (isocyanides and CO) ligands, instead of the strong σ-donor, phosphane and SH2 ligands. The strong σ-donor ligands are moved to the regions of strong and moderate trans-philicity. In the regions of strong and moderate trans-philicity are also found the N-donor (nitriles, Py, NH3, NH2−, N3− and NO2−) along with the SH−, SCN−, CN−, Br−, Cl− and OH2 ligands, while the O-donor (RCOO−, OX−, OCN− and OH−) ligands and F− are correctly placed in the rungs of weak trans-philicity.
In the trans-influence ladder the strong σ donor BH2−, B(OH)2−, H−, SnCl3−, Me−, t-Bu− and Ph− anionic ligands along with NH2−, OH−, SH−, CN−, Br−, N3− and SCN− ligands are placed in the rungs of very strong and strong trans-influence, in line with the experimentally established trans orienting series. The O-donor (RCOO−, OX−, OCN−) ligands along with NO2−, F−, Cl− and NCS− occupy the rungs with strong to moderate trans-influence. The C-donor (isocyanides and CO) along with the N-donor (nitriles, NH3, Py and N2) ligands occupy the rungs of weak trans-influence deviating from the experimentally established trans orienting series.
Correlations between the isotropic σiso(SO) X NMR shielding constants and the ligand electronic parameter PL
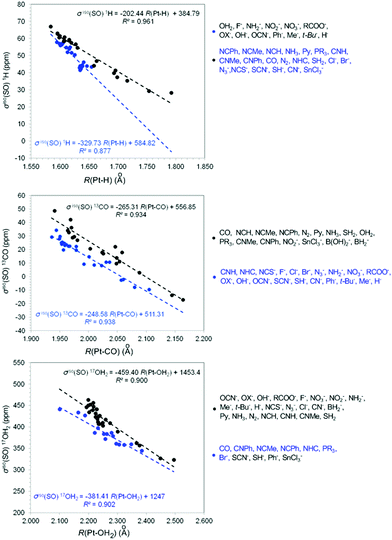
In order to scrutinize the underlying principles and the origin of trans philicity and threw some light on the still intriguing physics of the trans-influence phenomena we investigated relationships between the isotropic σiso(SO) X (X = 1H, 13CH3, 13CO, 15N, 17O and 35Cl) shielding constants and the well established ligand electronic parameters PL (a measure of the overall electron attracting or releasing quality of L).14 Representative linear plots of the σiso(SO) X (X = 1H, 13CO, 17OH2) vs. PL are shown in Fig. 1. Linear plots of the σiso(SO) X (X = 13CH3, 15NH2, 35Cl) vs. PL correlations are given in the ESI† (Fig. S1).Inspection of Fig. 1 and Fig. S1 (ESI†) reveals that accurate linear relationships are obtained for similar subsets of ligands L. In the σiso(SO) 1H vs. PL, σiso(SO) 17O vs. PL (Fig. 1) and σiso(SO) 15NH2vs. PL (Fig. S1, ESI†) the ligands are grouped into four families, in the σiso(SO) 13CO vs. PL, and σiso(SO) 35Cl vs. PL correlations (Fig. S1, ESI†) into two ligand families, while in the σiso(SO) 13CH3vs. PL, correlations into three ligand families.
Generally the O-, N-, C- and σ-donor ligands form their own families and in some cases are mixed with phosphane and S-donor ligands. Notice that the three and two ligand families result from the mixing of O- and N-donor ligands in the same family. Noteworthy in the σiso(SO) 1H vs. PL correlations (Fig. 1) and the σiso(SO) X (X = 13CH3, 15NH2 and 35Cl) vs. PL correlations (Fig. S1, ESI†) increase of the negative value of PL constant (increase of the electron releasing capacity of L) increases the downfield shifts of the σiso(SO) X NMR shielding constants. Conversely in the σiso(SO) X (X = 13CO and 17OH2) vs. PL correlations (Fig. 1) increase of the negative value of PL constant increases the upfield shifts of the σiso(SO) X NMR shielding constants. It should be noticed that the values of the PL constants, taken from ref. 14 are presented herein in from of a PL constants ladder (Chart 6).
 | ||
| Chart 6 P L constants ladder (values taken from ref. 14). | ||
The different NMR probe effects on the σiso(SO) X (X = 13CO and 17OH2) and σiso(SO) X (X = 1H, 13CH3, 15NH2 and 35Cl) could be explained by the synergism of the trans L and X ligands to balance the electron density transfer along the L–Pt–X framework through the σ- and π-subspaces that affects the X NMR shielding constants (compare the overall electron attracting or releasing quality of the X NMR probe given in Chart 6). The H− and CH3− NMR probes are strong σ-donors ligands. The same holds true for the NH2− and Cl− NMR probes with their σ-donor capacity enhanced by the weak π-donor capacity of these probes (these ligands are found at the top of the PL ladder). On the other hand H2O and CO NMR probes are weak σ-donors with the σ-donor capacity of the latter further diminished by its strong π-acceptor capacity (H2O and CO ligands are found at the low part of the PL ladder).
Correlations between the σiso(SO) X NMR descriptors of trans-philicity and the R(Pt–X) descriptors of trans-influence
To demonstrate weather trans-philicity is related with the trans-influence phenomenon we investigated relationships between the σiso(SO) X (X = 1H, 13CH3, 13CO, 15N, 17O and 35Cl) trans-philicity descriptors and the R(Pt–X) descriptors of trans-influence. The linear plots of the σiso(SO) X (X = 1H, 13CO, 17OH2, 13CH3, NH2 and 35Cl) vs. R(Pt–X). Correlations are shown in Fig. 2 and Fig. S2 (ESI†).Accurate correlation equations can be drawn from two ligand families for the σiso(SO) X (X = 1H, 13CO, 17OH2) vs. R(Pt–X) correlation, three ligand families for the σiso(SO) X vs. R(Pt–X) X = CH3 and NH2 correlation and four ligand families for the σiso(SO) 35Cl vs. R(Pt–Cl) correlations. The first family in the σiso(SO) X vs. R(Pt–X) X = H, CO, OH2 correlations involves most of the O-donor ligands mixed with some of the strong σ-donor and N-donor ligands, while the second ligand family involves all the remaining ligands.
In the σiso(SO) 13CH3vs. R(Pt–CH3) and σiso(SO) 15NH2vs. R(Pt–NH2) correlations the first family involves strong σ-donor BH2−, B(OH)2− and Ph− ligands mixed with OH−, OX−, OCN−, NO2−, NH2− and F− ligands. In the second family one finds N-donor ligands along with CN−, SCN−, SH− and SnCl3− ligands, while the third family involves the C-donor, nitriles, phosphanes, halides, H2S and H2O ligands.
The linear relationships for the σiso(SO) X (X = 1H, 13CO, 17OH2, 13CH3, NH2 and 35Cl) vs. R(Pt–X) correlations show that the increase of the R(Pt–X) descriptor of trans-influence increases the upfield shifts of the σiso(SO) X descriptors of trans-philicity.
Correlations between the σiso(SO) X NMR shielding constants and the WBI(Pt–X) and QPt electronic parameters
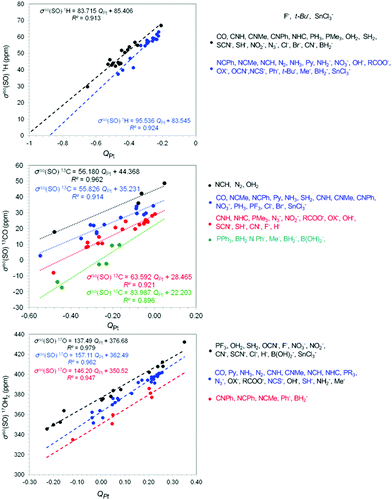
To probe further the electronic nature of trans philicity we investigated possible relationships of the σiso(SO) X (X = 1H, 13CH3, 13CO, 15NH2, 17OH2 and 35Cl) shielding constants and natural bond orbital (NBO) parameters, namely the Wiberg Bond Index of the Pt–X bond, WBI(Pt–X) and the natural atomic charge on Pt metal center QPt. Representative linear plots of the σiso(SO) X vs. WBI(Pt–X) and σiso(SO) X vs. QPt (X = H, CO, OH2) correlations are shown in Fig. 3 and 4 respectively. The linear plots of the σiso(SO) X vs. WBI(Pt–X) and σiso(SO) X vs. QPt (X = CH3, NH2, Cl) correlations are given in the ESI† (Fig. S3 and S4).Inspection of the linear plots given in Fig. 3 and Fig. S3 (ESI†) reveals that accurate linear equations can be drawn from distinct ligand families. For the σiso(SO) X vs. WBI(Pt–X) (X = H, CO, OH2, CH3) correlations accurate linear equations are obtained for three ligand families, while for the σiso (SO) X vs. WBI(Pt–X) (X = NH2, Cl) correlations from four ligand families. These ligand families are given in Fig. 3 and Fig. S3 (ESI†). It can also be seen that in all linear relationships the increase of WBI(Pt–X) (increase of the covalency of the Pt–X bond) increases the downfield shift of σiso(SO) X shielding constants. In this context the relation of the trans-philicity and trans-influence with the covalency of Pt–X bond demonstrates clearly the electronic origin of the two phenomena. Scheme 2 shows the 3D plots and composition of the natural bonding orbitals BD (Pt–X) and BD(L–Pt) for selected trans-Pt(PMe3)2(X)L (X = Cl, CO, CH3) complexes.
Taking into consideration that the primary determinant of trans-philicity is likely to be covalent contributions to bonding, whether they arise from σ donation or π back-donation, in the formation of the L → Pt dative bond electron density is transferred towards the coordination site trans to L. Interestingly, the σiso(SO) X shieldings linearly correlate with the calculated WBI(Pt–X) parameters, a measure of the covalency of Pt–X bond, demonstrating that the covalent bonding contributions to the Pt–X bond and the net charge transfer from the ligand L to Pt metal center are the key factors that manipulate trans-philicity. The bonding σ(Pt–X) NBOs are constructed from the interaction of the spmdn hybrid orbitals of the Pt(II) metal center with the s or spk hybrid orbitals of X and are described as σ(Pt–X) = c1(spmdn)Pt + c2(spk)X. The covalency of the σ(Pt–X) NBOs increases (higher WBI(Pt–X) values) by increasing the overlap of the (spmdn)Pt and (spk)X hybrid orbitals. Accordingly increase of the overlap results from the overlap of more diffuse (spmdn)Pt hybrid orbitals that exhibit higher d-orbital character illustrating the crucial role of 5d orbitals of Pt(II) in modulating the propagation of spin–orbit effects on the σiso(SO) shielding constants.
The trans-philicity descriptors are linearly correlated with the natural atomic charges on the Pt central atom, QPt, (Fig. 4 and Fig. S5, ESI†). For the σiso(SO) X vs. QPt correlations accurate linear equations are obtained from two, three or four ligand families, which are given in Fig. 4 and Fig. S4 (ESI†). At this point it is important to be noticed that in all correlations studied the grouping of the ligands into families is almost similar. The linear plots of the σiso(SO) X vs. QPt correlations show that the increase of electron density on the Pt central atom, QPt induces upfield shifts of σiso(SO) X shielding constants.
Conclusions
Quantitative trans-philicity ladders for a broad series of ligands (44 ligands) in square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes are built by the isotropic σiso(SO) X NMR trans-philicity descriptors. We also deployed the ligands under study in trans-influence sequences employing the R(Pt–X) structural descriptors. Accordingly the relation of trans-philicity with trans-influence validates the trans-philicity concept, as a unified term, avoiding confusion in the ambiguous use of the kinetic trans-effect and the structural trans-influence terms, often encountered in coordination chemistry. The relative trans-philicity strengths are expressed by the Δσiso(SO) X NMR trans-philicity metrics defined as the difference between the calculated σiso(SO) X NMR shielding constants for the complete set of ligands and the σiso(SO) X NMR shielding constant of the complex containing the ligand exerting the weakest trans-philicity phenomenon. Similarly the relative trans-influence strengths are expressed by the ΔR(Pt–X) structural metrics. Important results are summarized as follows.The NMR trans-philicity ladders built for square planar trans-[Pt(PMe3)2(X)L]n (n = 0, 1, 2; X = H, CO, CH3, NH2, OH2, Cl) complexes, involving a broad series of ligands (44 ligands) with diverse electronic features go roughly parallel to trans-influence ladders built by the ΔR(Pt–X) descriptors.
The NMR trans philicity descriptors depend on the nature of the NMR probe. The electronic effects of the NMR probes (σ-donor, σ-donor/π-acceptor, σ-donor/π-donor) tune the electron density transfer L → Pt → X pathways, thus affecting the σiso X trans-philicity descriptors. For the σ-donor NMR probes (1H, 13CH3 and 17OH2) the electron density transfer L → Pt → X takes place only through the σ-subspace (σ-channel), whereas for the σ-donor/π-acceptor 13CO and σ-donor/π-donor 15NH2 and 35Cl NMR probes the electron density transfer takes place through both the σ- and π-subspaces (σ- and π-channels). The synergic contribution of the σ- and π-electronic effects of L and X to balance the electron density transfer along the L → Pt → X framework are the crucial factors that manipulate trans-philicity. Generally very strong σ-donor ligands and σ-donor/π-acceptor ligands exert strong trans-philicity, σ-donor/π-donor ligands exert moderate trans-philicity, while weak σ-donors exert the weakest trans-philicity.
Excellent linear relationships between the isotropic σiso(SO) X shielding constants and the well established ligand electronic parameter PL and other popular electronic/structural descriptors related with the L–Pt–X bonding threw light on the underlying principles and the electronic origin of trans philicity.
The trans-philicity ladders constructed by the six different NMR probes go roughly parallel. Indeed all ladders are almost similar, but some minor deviations related with the position of L in the rungs of the trans-philicity ladders are observed.
Linear relationships between the σiso(SO) X trans-philicity descriptors and the R(Pt–X) descriptors of trans-influence demonstrate the relation of trans-philicity with trans-influence phenomenon, thus validating its use as a unified concept in the realm of inorganic, organometallic, coordination chemistry and catalysis. According to the linear relationships for the σiso(SO) X (X = 1H, 13CO, 17OH2, 13CH3, NH2 and 35Cl) vs. R(Pt–X) correlations the increase of the R(Pt–X) descriptor increases the upfield shifts of the σiso(SO) X shielding constants.
All linear relationships for the σiso(SO) X (X = 1H, 13CO, 17OH2, 13CH3, NH2 and 35Cl) vs. WBI(Pt–X) correlations showed that the increase of WBI(Pt–X) (increase of the covalency of the Pt–X bond) increases the downfield shift of σiso(SO) X shielding constants. Furthermore the increase of electron density on the Pt central atom increases the upfield shifts of the σiso(SO) X (X = 1H, 13CO, 17OH2, 13CH3, NH2 and 35Cl) shielding constants.
Conflicts of interest
There are no conflicts to declare.Notes and references
- R. G. Wilkins, The study of kinetics and mechanism of reactions of transition metal complexes, Allyn and Bacon, Boston, 1974, p. 229 Search PubMed.
- J. E. Huheey, Inorganic Chemistry, Harper and Row, New York, 2nd edn, 1978, pp. 498–503 Search PubMed.
- M. L. Tobe, Inorganic Reaction Mechanisms, Nelson, Southampton, Great Britain, 1972, vol. 191, pp. 58–88 Search PubMed.
- F. Basolo and R. G. Pearson, Prog. Inorg. Chem., 1962, 4, 381–453 CAS.
- J. D. Atwood and T. L. Brown, J. Am. Chem. Soc., 1975, 97, 3380 CrossRef CAS.
- J. D. Atwood and T. L. Brown, J. Am. Chem. Soc., 1976, 98, 3155 CrossRef CAS.
- J. D. Atwood and T. L. Brown, J. Am. Chem. Soc., 1976, 98, 3160 CrossRef CAS.
- D. L. Lichtemberger and T. L. Brown, J. Am. Chem. Soc., 1978, 100, 366 CrossRef.
- A. Pidcock, R. E. Richards and L. M. Venanzi, J. Chem. Soc. A, 1966, 1707–1710 RSC.
- C. A. Tsipis, Dalton Trans., 2019, 48, 1814–1822 RSC.
- G. Butler, J. Chatt, G. J. Leigh and C. J. Pickett, J. Chem. Soc., Dalton Trans., 1979, 113–116 RSC.
- J. Chatt, C. T. Kan, G. J. Leigh, C. J. Pickett and D. R. Stanley, J. Chem. Soc., Dalton Trans., 1980, 2032–2038 RSC.
- J. Chatt, Coord. Chem. Rev., 1982, 43, 337–347 CrossRef CAS.
- C. A. Tsipis, J. Comput. Chem., 2019, 40, 2550–2562 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, M. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 CrossRef CAS.
- M. Ernzerhof and G. Scuseria, J. Chem. Phys., 1999, 110, 5029 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158 CrossRef CAS.
- D. A. Pantazis, X.-Y. Chen, C. R. Landis and F. Neese, J. Chem. Theory Comput., 2008, 4, 908 CrossRef CAS PubMed.
- EMSL basis set exchange, accessed 08-01-2013, https://bse.pnl.gov/bse/portal.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899 CrossRef CAS.
- F. Weinhold, in The Encyclopedia of Computational Chemistry, ed. P. V. R. Schleyer, John Wiley & Sons, Chichester, UK, 1998 Search PubMed.
- R. Ditchfield, Mol. Phys., 1974, 27, 789–807 CrossRef CAS.
- J. Gauss, J. Chem. Phys., 1993, 99, 3629–3643 CrossRef CAS.
- E. van Lenthe, J. G. Snijders and E. J. Baerends, J. Chem. Phys., 1996, 105, 6505–6516 CrossRef CAS.
- E. van Lenthe, A. E. Ehlers and E. J. Baerends, J. Chem. Phys., 1999, 110, 8943–8953 CrossRef CAS.
- ADF 2012, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, accessed Feb 15, 2014, http://www.scm.com.
- (a) A. H. Greif, P. Hrobárik, V. Hrobáriková, A. V. Arbuznikov, J. Autschbach and M. Kaupp, Inorg. Chem., 2015, 54, 7199–7208 CrossRef CAS PubMed; (b) A. E. Shilov and G. B. Shul’pin, Chem. Rev., 1997, 97, 2879 CrossRef CAS PubMed.
- J. A. Labinger and J. E. Bercaw, Nature, 2002, 417, 507 CrossRef CAS PubMed.
- A. S. Goldman and K. I. Goldberg, ACS Symp. Ser., 2004, 885, 1–43 CrossRef CAS.
- R. Waterman, Organometallics, 2013, 32, 7249–7263 CrossRef CAS.
- H. Braunschweig, P. Brenner, R. D. Dewhurst, F. Guethlein, J. O. C. Jimenez-Halla, K. Radacki, J. Wolf and L. Zöllner, Chem. – Eur. J., 2012, 18, 8605–8609 CrossRef CAS.
- Y.-P. Ho, S. C. F. Au-Yeung and K. K. W. To, Med. Res. Rev., 2003, 23, 633–655 CrossRef CAS PubMed.
- Y. Jung and S. J. Lippard, Chem. Rev., 2007, 107, 1387–1407 CrossRef CAS PubMed.
- H.-K. Liu and P. J. Sadler, in NMR of Biomolecules: Towards Mechanistic Systems Biology, ed. I. Bertini, K. S. McGreevy and G. Parigi, Wiley-VCH Verlag GmbH & Co. KGaA, 2012, ch. 16, pp. 282–296 Search PubMed.
- T. G. Appleton, C. Clark and L.-E. Manzer, Coord. Chem. Rev., 1973, 10, 335–422 CrossRef CAS.
- Z. Chval, M. Sip and J. V. Burda, J. Comput. Chem., 2008, 29, 2370–2381 CrossRef CAS.
- J. Chatt and B. L. Shaw, J. Chem. Soc. Resumed, 1962, 5075 RSC.
- L. Abis, A. Sen and J. Halpern, J. Am. Chem. Soc., 1978, 100, 2915–2916 CrossRef CAS.
- C. M. Jensen and W. C. Trogler, J. Am. Chem. Soc., 1986, 108, 723–729 CrossRef CAS.
- D. L. Packett and W. C. Trogler, Inorg. Chem., 1988, 27, 1768–1775 CrossRef CAS.
- D. Ramprasad, H. J. Yue and J. A. Marsella, Inorg. Chem., 1988, 27, 3151–3155 CrossRef CAS.
- A. H. Greif, P. Hrobárik and M. Kaupp, Chem. – Eur. J., 2017, 23, 9790–9803 CrossRef CAS PubMed.
- M. P. Mitoraj and A. Michalak, Inorg. Chem., 2010, 49, 578–582 CrossRef CAS PubMed.
- T. R. Kégl, L. Kollár and T. Kégl, Adv. Chem., 2016, 1–7 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: σiso(SO) X NMR shielding constants (X = H, CH3, CO, NH2, OH2, Cl) calculated at the SO-ZORA level of theory (Tables S1–S8); linear plots of the correlations between the σiso(SO) X NMR shielding constants (X = CH3, NH2, Cl) and PL, R(Pt–X), WBI(Pt–X) and QPt (Fig. S1–S4). See DOI: 10.1039/d0nj01336f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2020 |