Di-butyltin(iv) complexes with azo-carboxylates: synthesis, characterization, crystal structures and their anti-diabetic assay†
Abstract
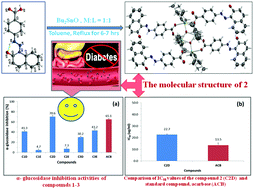
Dibutyltin(IV) complexes 1–3 were synthesized by the reaction of either dibutyltin(IV) oxide or dibutyltin(IV) dichloride with azo-carboxylic acid ligands viz. 2/4-(2-hydroxynaphthylazo)-benzoic acids in different stoichiometric reaction ratios. The complexes were characterized by elemental analysis and UV, IR and multinuclear [1H, 13C and 119Sn]-NMR spectroscopy. The geometry and mode of coordination around tin atoms in the complexes in the solid state were determined by X-ray crystal structure analysis. Complex 1 exhibited a dinuclear structure with a distorted square-pyramidal geometry around each Sn atom. Compound 2 showed a bis[dicarboxylatotetraorganodistannoxane] type structure {[R2Sn(O2CR′)]2O}2 that contained a centrosymmetric Sn2O4 core in which the Sn2O2 ring was connected to the exo-cyclic tin atom through μ3-oxo O-atoms. The coordination geometry around exo- and endo-cyclic tin atoms was intermediate between square-pyramidal and trigonal bipyramidal and distorted octahedral, respectively. In 3, the coordination sphere around the tin atom adopted a skew-trapezoidal bipyramidal geometry. The 119Sn NMR study suggested a 5-coordinate structure in complexes 1 and 3 in the solution state, while in 2 the geometry around exo- and endo-cyclic tin atoms was found to be a five and six coordinate structure, respectively. Thus, the solid state structure of 1 and 2 is retained in solution, while in 3 the solid state structure is dissociated. The anti-diabetic activity of the complexes was also studied and the results of the assays showed significant activity of compound 2 compared to 1 and 3.


 Please wait while we load your content...
Please wait while we load your content...