Effects of oxidation treatment by KClO3/H2SO4 systems on the chemical, crystal and microscopic structures of polyacrylonitrile fibers
Abstract
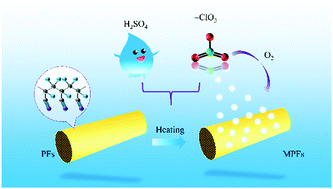
The effects of various KClO3 concentrations and durations on polyacrylonitrile (PAN) fibers were investigated. The oxidation treatment of PAN fibers (PFs) in a KClO3/H2SO4 system is a new pretreatment method for carbon fiber oxidation stabilization. Wettability (droplet shape), chemical structures (Fourier transform infrared spectroscopy [FT-IR]), crystal structures (wide-angle X-ray diffraction), microscopic structures (scanning electron microscopy), and chemical components (X-ray photoelectron spectroscopy) were analyzed. The PFs had the smallest contact angle and the best wettability when the KClO3 concentration was 15 wt%. FT-IR analysis showed that the absorption peak of the fibers was the lowest when the KClO3 concentration was 15 wt%. The peak intensities of MPFs-15-60 and MPFs-15-90 at 2θ = 17° and 2θ = 29°, respectively, were close and lower than the peak intensities of others. The number of grooves present on the fiber surface decreased when the KClO3 concentration was 15 wt%. As the KClO3 concentration increased, the elemental C content decreased and the elemental O content increased. MPFs-15-60 had a low C content and the highest O content, indicating that it had the highest degree of oxidation. The best sample for PF oxidation treatment with KClO3 was MPFs-15-60. The optimal processing conditions were a KClO3 concentration of 15 wt% and duration of 60 min.


 Please wait while we load your content...
Please wait while we load your content...