Coumarin–tetraphenylethylene regioisomers: synthesis, photophysical and aggregation-induced emission properties†
Abstract
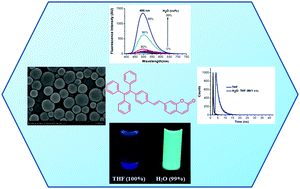
Coumarin–tetraphenylethylene (CTPE) regioisomers with different linkage types (single-bond, vinyl, and acetylene) and substitution positions (coumarin C5, C6, C7) were synthesized and characterized using 1H NMR, 13C NMR, and high-resolution mass spectroscopy. The effects of substitution position and conjugation in CTPEs 1–9 on absorption, fluorescence, and aggregation-induced emission enhancement were explored. Electronic absorption and emission spectra indicate that CTPEs with C7 substitution are red-shifted compared to those substituted at C5 or C6. CTPEs 1–9 form aggregates in tetrahydrofuran/water (1 : 99, v/v) and exhibit aggregation-induced emission. Nanoaggregates were characterized using scanning electron microscopy and dynamic light scattering. The structure of CTPE 1 was confirmed by single crystal X-ray diffraction analysis.


 Please wait while we load your content...
Please wait while we load your content...