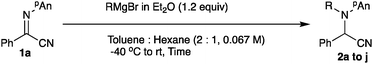An umpolung reaction of α-iminonitriles and its application to the synthesis of aminomalononitriles†
Makoto
Shimizu
 *ab,
Yuki
Furukawa
b,
Isao
Mizota
b and
Yusong
Zhu
*ab,
Yuki
Furukawa
b,
Isao
Mizota
b and
Yusong
Zhu
 a
a
aSchool of Energy Science and Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu Province, China
bDepartment of Chemistry for Materials, Graduate School of Engineering, Mie University, Tsu, Mie 514-8507, Japan. E-mail: mshimizu@chem.mie-u.ac.jp
First published on 20th November 2019
Abstract
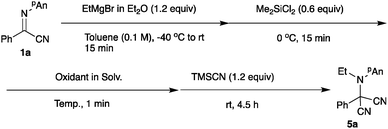
An umpolung N-alkylation reaction of α-iminonitriles with Grignard reagents affords the corresponding N-alkylated α-aminonitriles in good yields. Subsequent oxidation and cyanation of the N-alkylated products proceeds effectively to give aminomalononitriles in good yields, and the presence of dichlorodimethylsilane as an additive is crucial for obtaining the optimum yield. Furthermore, an electrophilic addition reaction of the intermediate halomagnesium vinylideneamide is shown to give the alkylated and acylated products in good yields.
1 Introduction
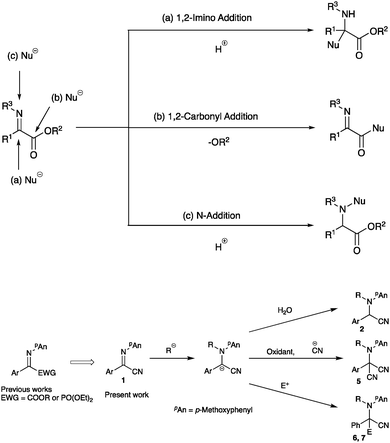
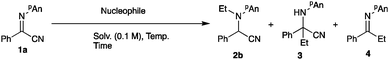
The intriguing reactivity of α-iminoesters has broadened the utility of imino compounds as amination reagents and precursors to α-aminoesters via an addition to the imino carbon.1–3 Three reactive positions are available for the addition of nucleophiles to α-iminoesters: the first is the imino carbon, the second is the ester carbonyl group, and the third is the imino nitrogen. The reactions involving the addition to the nitrogen atom of the imine moiety are categorized as umpolung reactions because the reversal of the polarity at the imine part is crucial for this type of reaction. We have already disclosed several intriguing reactions based on the umpolung reactivity of α-iminoesters,3 demonstrating that the alkoxycarbonyl3a–e and dialkoxyphosphoryl4 groups are useful as electron-withdrawing groups next to the imine moiety for the umpolung N-alkylations. In addition to these groups, we were interested in cyano derivatives, since the cyano moiety can be readily transformed into several useful functional groups such as amines, amides, and carboxylic acids.5–8 In this paper we examined the umpolung reactions of α-iminonitriles (Scheme 1).2 Results and discussion
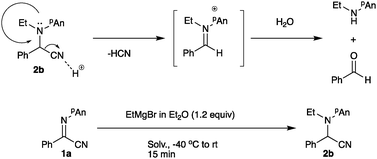
The starting α-iminonitrile 1 was readily prepared according to the procedure reported by Masson and Zhu.9 First, the conditions for the ethylation reaction of α-iminonitrile 1a including the ethylation reagent, solvent, time, and temperature were examined. Table 1 summarizes the results.| Entry | Nucleophile (equiv.) | Solv. | Temp. (°C) | Time (min) | Yielda (%) | ||
|---|---|---|---|---|---|---|---|
| 2b | 3 | 4 | |||||
a Isolated yield.
b Recovery of the starting material (95%).
c Recovery of the starting material (89%).
d Recovery of the starting material (16%).
e Recovery of the starting material (46%).
f The reaction was carried out in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nhexane = 2 nhexane = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.67 M). 1 (0.67 M).
|
|||||||
| 1 | EtMgBr/Et2O (1.2) | PhCH3 | −40 to rt | 15 | 76 | — | — |
| 2 | EtMgBr/THF (1.2) | PhCH3 | −40 to rt | 15 | 3 | 14 | 77 |
| 3 | Et2AlCl/nhex (2.0) | EtCN | −78 to rt | 300 | 0b | — | — |
| 4 | Et2Zn/nhex (2.0) | PhCH3 | −78 to rt | 300 | 0c | — | — |
| 5 | EtMgBr/Et2O (1.2) | PhCH3 | −40 | 60 | 63 | — | — |
| 6 | EtMgBr/Et2O (1.2) | PhCH3 | −40 to 0 | 60 | 62 | — | — |
| 7 | EtMgBr/Et2O (1.2) | CH2Cl2 | −40 to rt | 15 | 52 | 9 | 18 |
| 8 | EtMgBr/Et2O (1.2) | Et2O | −40 to rt | 15 | 52d | 6 | 2 |
| 9 | EtMgBr/Et2O (1.2) | DME | −40 to rt | 15 | 0 | — | 55 |
| 10 | EtMgBr/Et2O (1.2) | nhex | −40 to rt | 15 | 43e | — | — |
| 11f | EtMgBr/Et2O (1.2) | PhCH3/nhex | −40 to rt | 15 | 74 | — | — |
One of the side reactions associated with the use of α-iminonitrile 1 as the electrophile is the replacement of the cyano group with a nucleophile, since the cyano group is known to be a relatively good leaving group in many organic transformations.10 This side reaction was the major pathway, when the addition was conducted with the Grignard reagent prepared in THF or when the reaction was carried out in DME (entries 2 and 9). Among the ethylation reagents examined, ethylmagnesium bromide added to the imino nitrogen, whereas diethylaluminum chloride and diethylzinc were not effective as the nucleophiles (entries 3 and 4). Regarding the solvent, EtCN did not promote the present N-ethylation, while N-ethylation proceeded in toluene, dichloromethane, ether, and nhexane. The N-ethylation product 2b was not stable under the purification conditions on silica gel TLC;11 however, silica gel column chromatography using an eluent containing triethylamine (10 v/v%) was found to be suitable for purification. In terms of the clearness of the reaction medium, the best result was obtained when the reaction was carried out in toluene–nhexane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (entry 11), and the product was purified as summarized in Table 2.
1) (entry 11), and the product was purified as summarized in Table 2.
Under the best N-alkylation conditions found for the substrate 1a (entry 3, Table 2), a variety of Grignard reagents were subjected to N-alkylation, and Table 3 summarizes the results.
Primary alkyl Grignard reagents were added to the nitrogen atom in moderate to good yields (entries 2–6), whereas their sterically bulky secondary alkyl counterparts underwent the addition sluggishly. In the present N-addition reaction (entries 7 and 8), methyl, phenyl, and benzyl derivatives did not give the desired products at all (entries 1, 9, and 10).12 Other α-iminonitriles 1b to 1j were also examined for the N-ethylation reaction, and Table 4 summarizes the results.
| Entry | 1: R | Temp. (°C) | Time (min) | 2: yielda (%) |
|---|---|---|---|---|
| a Isolated yield. b The reaction was carried out in toluene (0.033 M). c EtMgBr in Et2O (1.5 equiv.) was used. | ||||
| 1 | 1a: Ph | −40 to rt | 15 | 2b: 85 |
| 2 | 1b: 4-BrC6H4 | −10 to rt | 25 | 2k: 67 |
| 3 | 1c: 4-MeOC6H4 | −20 to rt | 35 | 2l: 52 |
| 4 | 1d: 2-BrC6H4 | −40 to rt | 15 | 2m: 85 |
| 5b | 1e: 2-Pyridyl | −78 | 15 | 2n: 52 |
| 6b | 1f: 2-Furyl | −78 | 15 | 2o: 82 |
| 7c | 1g: 2-Thienyl | −15 | 45 | 2p: 49 |
| 8b | 1h: 1-Naphthyl | −20 to rt | 15 | 2q: 72 |
| 9 | 1i: 2-Naphthyl | −40 to rt | 15 | 2r: 78 |
| 10 | 1j: Cy | −40 to rt | 25 | 2s: 31 |
Almost all the substrates examined here participated in the present N-alkylation reaction to give the desired product 2 in moderate to good yields. Electron-withdrawing or electron-donating groups on the aromatic carbons were tolerated and a sterically congesting 2-bromo group did not decrease the product yield (entries 2–4). Heteroaromatic substituents such as 2-pyridyl, 2-furyl, and 2-thienyl were also tolerated (entries 5–7). We next examined the subsequent oxidation/cyanation reactions, and Table 5 summarizes the results.
| Entry | R4−nSiCln (equiv.) | Temp. 1 (°C) | Time (min) | Temp. 2 (°C) | Yielda (%) | |
|---|---|---|---|---|---|---|
| 5a | 6 | |||||
| a Isolated yield. | ||||||
| 1 | — | — | — | 0 | 49 | — |
| 2 | — | — | — | −20 | 49 | — |
| 3 | — | — | — | −40 | 44 | 16 |
| 4 | Me3SiCl (1.2) | −40 | 15 | 0 | 44 | 10 |
| 5 | Me3SiCl (1.2) | 0 | 15 | 0 | 40 | 14 |
| 6 | Me3SiCl (1.2) | 0 | 60 | 0 | 46 | 13 |
| 7 | Me2SiCl2 (1.2) | −40 | 15 | 0 | 46 | 10 |
| 8 | Me2SiCl2 (1.2) | 0 | 15 | 0 | 45 | Trace |
| 9 | Me2SiCl2 (1.2) | 0 | 60 | 0 | 45 | — |
| 10 | Me2SiCl2 (0.6) | 0 | 15 | 0 | 55 | 5 |
| 11 | Me2SiCl2 (0.2) | 0 | 15 | 0 | 50 | 16 |
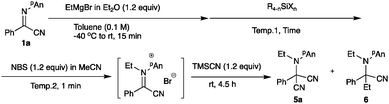
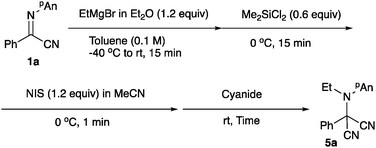
As mentioned above, since a slight excess of Grignard reagent was needed to effectively accomplish the N-ethylation, the formation of the diethylated product was observed in several cases. To supress the formation of the undesired diethylated product 6, we examined a series of additives and oxidation reagents. Although the use of Me3SiCl did not improve the product yield noticeably (entries 4–6), the presence of Me2SiCl2 (0.6 equiv.) increased the yield of the desired product 5a up to 55% (entry 10). Table 6 summarizes the screening of the oxidation reagents. As can be seen, NBS (1.2 equiv.), DBDMH (0.6 equiv.), NCS (1.2 equiv.), and NIS (1.2 equiv.) in MeCN could be used, with the latter affording the best result (entry 12). We next examined the cyanation reagent, and Table 7 summarizes the results.
| Entry | Oxidant (equiv.) | Solv. | Temp. (°C) | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | NBS (1.2) | MeCN | 0 | 55 |
| 2 | NBS (1.5) | MeCN | 0 | 51 |
| 3 | NCS (1.2) | MeCN | 0 | 50 |
| 4 | NIS (1.2) | MeCN | 0 | 61 |
| 5 | DBDMH (0.6) | MeCN | 0 | 55 |
| 6 | BPO (1.2) | MeCN | 0 | 13 |
| 7 | DMP (1.2) | — | 0 | 12 |
| 8 | DDQ (1.2) | MeCN | 0 | 35 |
| 9 | NIS (1.2) | CH2Cl2 | 0 | 44 |
| 10 | NIS (1.2) | DMF | 0 | 37 |
| 11 | NIS (1.2) | — | 0 | 34 |
| 12 | NIS (1.2) | MeCN | −40 | 58 |
| 13 | NIS (1.2) | MeCN | 40 | 44 |
| Entry | Cyanide (equiv.) | Time (h) | Yielda (%) |
|---|---|---|---|
| a Isolated yield. b Et3N (1.2 equiv.) was used as an additive. c Et3N (2.0 equiv.) was used as an additive. d Et3N (3.0 equiv.) was used as an additive. | |||
| 1 | TMSCN (1.2) | 4.5 | 61 |
| 2 | TMSCN (1.2) | 1.5 | 60 |
| 3 | TMSCN (1.2) | 3.0 | 63 |
| 4 | Et3AlCN (1.2) | 3.0 | 53 |
| 5 | TBSCN (1.2) | 3.0 | 64 |
| 6 | nBu3SnCN (1.2) | 3.0 | 72 |
| 7 | nBu3SnCN (1.5) | 3.0 | 65 |
| 8 | nBu3SnCN (2.0) | 3.0 | 63 |
| 9b | Acetone cyanohydrin (1.2) | 3.0 | 53 |
| 10c | Acetone cyanohydrin (2.0) | 3.0 | 66 |
| 11d | Acetone cyanohydrin (3.0) | 3.0 | 65 |
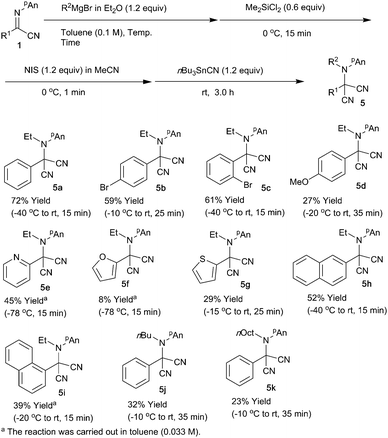
Trimethylsilyl cyanide and acetone cyanohydrin13 were applicable; however, the best result was obtained when the reaction was carried out with tributyltin cyanide (entry 6).14 Under the optimized conditions, various α-iminonitriles 1 were subjected to the present N-alkylation/oxidation/cyanation reaction, and Scheme 2 summarizes the results.
para-Substitution of aromatic carbon with the electron-donating methoxy group considerably decreased the product yield, whereas the electron-withdrawing bromo group in the ortho or para positions did not affect the reaction outcome, giving the desired products 5b and c in good yields. 2-Pyridyl and 1- and 2-naphthyl derivatives also gave the corresponding malononitriles 5e, h, and i in moderate yields, respectively. In contrast, in the butylation or octylation/oxidation/cyanation reactions, the reaction proceeded sluggishly, giving the desired products 5j and k in low yields. We next examined the use of an intermediate N-addition product for the subsequent C–C bond formations, and Scheme 3 shows the results.
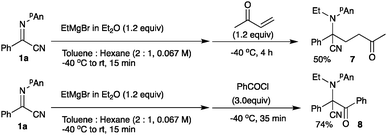
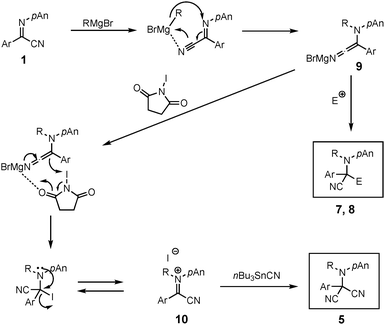
Treatment of the N-ethylation intermediate with MVK or benzoyl chloride gave the corresponding addition products 7 or 8 in 50% or 74% yield, respectively, indicating that this type of three-component coupling reaction is also possible using α-iminonitriles 1. However, the addition of benzaldehyde and methyl iodide did not proceed, giving exclusively N-ethylation product 2b. On the basis of the above results and our previous investigations,3 we propose the following reaction pathways (Scheme 4). First, the Grignard reagent attacks at the nitrogen atom to form bromomagnesium amide 9, which is oxidized with NIS to form the iminium salt 10. This iminium salt 10 is attacked by nBu3SnCN to give α-aminomalononitrile 5. Products 7 or 8 are formed via the addition of bromomagnesium amide 9 with an electrophile such as MVK or benzoyl chloride.
3 Conclusions
In conclusion, α-iminonitriles readily underwent the N-alkylation reaction with Grignard reagents, giving the corresponding α-aminonitriles in good yields. Subsequent oxidation/cyanation of the N-alkylated products was conducted with NIS/nBu3SnCN to produce the malononitrile derivatives in moderate to good yields. We also found that the N-alkylation intermediate could be used for further C–C bond formations with MVK and benzoyl chloride.15 The present umpolung reaction of α-iminonitriles offers a new approach for the synthesis of α-aminonitriles and aminomalononitriles.164 Experimental
General aspects
Melting point (mp) measurements were performed using a YAMATO MP-21 instrument and are uncorrected. Infrared spectra were recorded using a JASCO FT/IR-460 plus spectrometer. 1H NMR and 13C NMR spectra were recorded using a JEOL ECX-400P, or a JEOL A-500 spectrometer using tetramethylsilane as an internal standard. Mass spectra were recorded using a JEOL MS-700D spectrometer. Acetonitrile (MeCN) and propionitrile (EtCN) were distilled from phosphorus pentaoxide and then from calcium hydride, and stored over molecular sieves 4 Å. Diethylether (Et2O) was purified using a Glass Contour Organic Solvent Purification System from Nikko Hansen & Co., Ltd. Toluene, nhexane, dichloromethane (CH2Cl2), and dimethyl formamide (DMF) were distilled from calcium hydride and stored over molecular sieves 4 Å. Dimethoxyethane (DME) was distilled from calcium hydride and then from copper(I) chloride, and stored over sodium. Purification of products was performed by column chromatography on silica gel (Kanto Silica Gel 60N) and/or preparative TLC on silica gel (Merck Kiesel Gel GF254 or Wako Gel B-5F).Synthesis of (Z)-N-(4-methoxyphenyl)benzimidoylcyanide: general procedure for the synthesis of α-iminonitrile 1a
In a 100 mL two-necked round-bottomed flask equipped with a magnetic stirring bar was placed a solution of p-anisidine (1.82 g, 14.8 mmol) in MeCN (15.0 mL) under an argon atmosphere. To it were added benzaldehyde (1.50 mL, 14.8 mmol) and TMSCN (2.04 mL, 16.3 mmol). After the reaction mixture was stirred at room temperature for 1 h, IBX (4.57 g, 16.3 mmol) and nBu4NBr (5.25 g, 16.3 mmol) were added successively at 0 °C. After being stirred at 0 °C for 20 h, the mixture was filtered through a pad of Celite with the aid of nhexane. The solvent was evaporated, and the residue was purified by silica gel column chromatography (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 10
ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 1a.
1) to give the title compound 1a.
Yield 70% (2.45 g); m.p. 77–78 °C; yellow solid; Rf = 0.20 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 10
ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 6.97–7.03 (m, 2H), 7.25–7.36 (m, 2H), 7.49–7.58 (m, 3H), 8.12–8.15 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.6, 114.4, 123.0, 127.9, 128.9, 132.3, 134.1, 136.8, 141.7, 159.5; IR (KBr) 2963, 2837, 2217, 1607, 1503, 1275, 1245, 1107, 826, 685 cm−1. These spectra were identical to the reported data.9
1); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 6.97–7.03 (m, 2H), 7.25–7.36 (m, 2H), 7.49–7.58 (m, 3H), 8.12–8.15 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.6, 114.4, 123.0, 127.9, 128.9, 132.3, 134.1, 136.8, 141.7, 159.5; IR (KBr) 2963, 2837, 2217, 1607, 1503, 1275, 1245, 1107, 826, 685 cm−1. These spectra were identical to the reported data.9
(Z)-4-Bromo-N-(4-methoxyphenyl)benzimidoylcyanide 1b
Yield 7% (326.0 mg); m.p. 103–106 °C; yellow solid; Rf = 0.70 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 4
ethyl acetate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.86 (s, 3H), 6.97–7.01 (m, 2H), 7.34–7.38 (m, 2H), 7.63–7.67 (m, 2H), 7.97–8.00 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.5, 114.5, 123.3, 127.2, 129.2, 132.2, 133.1, 135.2, 141.3, 159.8; IR (KBr) 2957, 2930, 2214, 1611, 1505, 1262, 1163, 825, 712, 695 cm−1; HRMS (EI) calcd for C15H11BrN2O (M)+ 314.0055, found 314.0045.
1); 1H NMR (400 MHz, CDCl3) δ 3.86 (s, 3H), 6.97–7.01 (m, 2H), 7.34–7.38 (m, 2H), 7.63–7.67 (m, 2H), 7.97–8.00 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.5, 114.5, 123.3, 127.2, 129.2, 132.2, 133.1, 135.2, 141.3, 159.8; IR (KBr) 2957, 2930, 2214, 1611, 1505, 1262, 1163, 825, 712, 695 cm−1; HRMS (EI) calcd for C15H11BrN2O (M)+ 314.0055, found 314.0045.
(Z)-4-Methoxy-N-(4-methoxyphenyl)benzimidoylcyanide 1c
Yield 42% (1.76 g); m.p. 59–60 °C; yellow solid; Rf = 0.56 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 4
ethyl acetate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 3.89 (s, 3H), 6.96–7.02 (m, 4H), 7.24–7.28 (m, 2H), 8.05–8.09 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 55.5, 111.7, 114.3, 114.4, 122.7, 127.0, 129.8, 136.6, 142.1, 159.0, 163.1; IR (KBr) 2962, 2934, 2219, 1605, 1503, 1249, 1109, 840, 707, 666 cm−1; HRMS (EI) calcd for C16H14N2O2 (M)+ 266.1055, found 266.1054.
1); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 3.89 (s, 3H), 6.96–7.02 (m, 4H), 7.24–7.28 (m, 2H), 8.05–8.09 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 55.5, 111.7, 114.3, 114.4, 122.7, 127.0, 129.8, 136.6, 142.1, 159.0, 163.1; IR (KBr) 2962, 2934, 2219, 1605, 1503, 1249, 1109, 840, 707, 666 cm−1; HRMS (EI) calcd for C16H14N2O2 (M)+ 266.1055, found 266.1054.
(Z)-2-Bromo-N-(4-methoxyphenyl)benzimidoylcyanide 1d
Yield 33% (1.52 g); m.p. 59–61 °C; yellow solid; Rf = 0.58 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 4
ethyl acetate = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 3.87 (s, 3H), 7.00–7.03 (m, 2H), 7.35–7.48 (m, 4H), 7.69–7.72 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.7, 114.5, 121.7, 123.3, 127.8, 131.2, 132.1, 134.1, 135.5, 135.8, 141.0, 160.1.; IR (KBr) 2961, 2942, 2211, 1607, 1502, 1278, 1107, 835, 709, 676 cm−1; HRMS (EI) calcd for C15H11BrN2O (M)+ 314.0055, found 314.0053.
1); 1H NMR (400 MHz, CDCl3) δ 3.87 (s, 3H), 7.00–7.03 (m, 2H), 7.35–7.48 (m, 4H), 7.69–7.72 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.7, 114.5, 121.7, 123.3, 127.8, 131.2, 132.1, 134.1, 135.5, 135.8, 141.0, 160.1.; IR (KBr) 2961, 2942, 2211, 1607, 1502, 1278, 1107, 835, 709, 676 cm−1; HRMS (EI) calcd for C15H11BrN2O (M)+ 314.0055, found 314.0053.
(Z)-N-(4-Methoxyphenyl)picolimidoylcyanide 1e
Yield 52% (1.91 g); m.p. 73–74 °C; orange solid; Rf = 0.14 (toluene); 1H NMR (400 MHz, CDCl3) δ 3.87 (s, 3H), 6.99–7.03 (m, 2H), 7.42–7.46 (m, 1H), 7.48–7.52 (m, 2H), 7.82–7.86 (m, 1H), 8.23–8.25 (m, 1H), 8.79–8.84 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 55.5, 112.0, 114.4, 121.5, 124.0, 125.8, 136.5, 136.8, 140.5, 149.7, 152.5, 160.2; IR (KBr) 2982, 2946, 2218, 1613, 1508, 1248, 1176, 772, 742, 664 cm−1; HRMS (EI) calcd for C14H11N3O (M)+ 237.0902, found 237.0912.(Z)-N-(4-Methoxyphenyl)furan-2-carbimidoylcyanide 1f
Yield 38% (1.38 g); m.p. 97–98 °C; orange solid; Rf = 0.22 (toluene); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 6.62–6.64 (m, 1H), 6.96–7.00 (m, 2H), 7.20–7.21 (m, 1H), 7.35–7.39 (m, 2H), 7.67–7.67 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.0, 112.8, 114.5, 117.4, 123.5, 125.6, 141.0, 146.9, 150.1, 159.7; IR (KBr) 2972, 2951, 2217, 1611, 1503, 1255, 1108, 1082, 912, 753 cm−1; HRMS (EI) calcd for C13H10N2O2 (M)+ 226.0742, found 226.0741.(Z)-N-(4-Methoxyphenyl)thiophen-2-carbimidoylcyanide 1g
Yield 29% (1.08 g); m.p. 95–96 °C; orange solid; Rf = 0.44 (toluene); 1H NMR (400 MHz, CDCl3) δ 3.86 (s, 3H), 6.96–7.01 (m, 2H), 7.16–7.21 (m, 1H), 7.32–7.37 (m, 2H), 7.56–7.58 (m, 1H), 7.76–7.77 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.2, 114.4, 123.4, 128.2, 130.4, 131.9, 132.4, 141.0, 141.5, 159.6; IR (KBr) 2967, 2947, 2213, 1607, 1501, 1249, 1106, 927, 838, 634 cm−1; HRMS (EI) calcd for C13H10N2OS (M)+ 242.0514, found 242.0505.(Z)-N-(4-Methoxyphenyl)-1-naphthimidoylcyanide 1h
Yield 43% (1.97 g); m.p. 130–133 °C; orange solid; Rf = 0.58 (toluene); 1H NMR (400 MHz, CDCl3) δ 3.87 (s, 3H), 7.00–7.05 (m, 2H), 7.36–7.40 (m, 2H), 7.56–7.66 (m, 3H), 7.92–7.94 (m, 1H), 8.01–8.03 (m, 1H), 8.20–8.21 (m, 1H), 9.11–9.26 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.0, 112.3, 114.5, 117.4, 122.7, 124.8, 125.4, 126.8, 128.3, 128.9, 131.2, 133.1, 134.1, 159.4; IR (KBr) 2982, 2942, 2221, 1604, 1509, 1256, 1166, 841, 753, 633 cm−1; HRMS (EI) calcd for C19H14N2O (M)+ 286.1106, found 286.1115.(Z)-N-(4-Methoxyphenyl)-2-naphthimidoylcyanide 1i
Yield 67% (2.65 g); m.p. 100–101 °C; yellow solid; Rf = 0.47 (toluene); 1H NMR (400 MHz, CDCl3) δ 3.85 (s, 3H), 6.99–7.02 (m, 2H), 7.36–7.39 (m, 2H), 7.54–7.61 (m, 2H), 7.87–7.98 (m, 3H), 8.21–8.26 (m, 1H), 8.54–8.57 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 55.5, 111.8, 114.4, 122.8, 123.1, 127.1, 127.8, 128.4, 128.8, 129.2, 130.4, 131.8, 132.7, 135.1, 136.7, 141.8, 159.5; IR (KBr) 3000, 2968, 2215, 1606, 1505, 1255, 1169, 837, 763, 619 cm−1; HRMS (EI) calcd for C19H14N2O (M)+ 286.1106, found 286.1093.(Z)-N-(4-Methoxyphenyl)cyclohexanecarbimidoylcyanide 1j
Yield 23% (665.2 mg); m.p. 51–52 °C; yellow solid; Rf = 0.43 (toluene); 1H NMR (400 MHz, CDCl3) δ 1.21–1.59 (m, 5H), 1.72–1.76 (m, 1H), 1.86–1.90 (m, 2H), 2.04–2.08 (m, 2H), 2.57–2.76 (m, 1H), 3.82 (s, 3H), 6.90–6.94 (m, 2H), 7.06–7.10 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 25.4, 25.6, 29.4, 47.0, 55.4, 111.8, 114.3, 122.0, 142.0, 146.3, 158.8; IR (KBr) 2937, 2854, 2211, 1608, 1507, 1416, 1246, 1168, 839, 634 cm−1; HRMS (EI) calcd for C15H18N2O (M)+ 242.1419, found 242.1420.Synthesis of 2-[ethyl(4-methoxyphenyl)amino]-2-phenylacetonitrile: general procedure for the synthesis of α-aminonitrile 2b
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed (Z)-N-(4-methoxyphenyl)benzimidoylcyanide (47.3 mg, 0.20 mmol) in toluene (2.0 mL) and nhexane (1.0 mL) at −40 °C. To it was added EtMgBr (0.13 mL, 0.24 mmol, 1.80 M in Et2O). After the mixture was stirred for 15 min at room temperature, the reaction was quenched with sat aq NaHCO3 (5 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et3N = 10
Et3N = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 2b.
1) to give the title compound 2b.
Yield 89% (47.4 mg); m.p. 46–47 °C; yellow solid; Rf = 0.61 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.00 (dd, J = 6.9, 6.9 Hz, 3H), 2.99 (dq, J = 6.9, 13.3 Hz, 1H), 3.21 (dq, J = 6.9, 13.3, 1H), 3.77 (s, 3H), 5.39 (s, 1H), 6.84–6.87 (m, 2H), 7.05–7.07 (m, 2H), 7.38–7.40 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.1, 43.5, 55.4, 61.2, 114.4, 117.0, 123.7, 127.5, 128.7, 128.7, 134.0, 140.4, 156.1; IR (KBr) 2983, 2831, 2228, 1880, 1615, 1512, 1448, 1254, 1225, 1035 cm−1; HRMS (EI) calcd for C17H18N2O (M)+ 266.1419, found 266.1423.
1); 1H NMR (400 MHz, CDCl3) δ 1.00 (dd, J = 6.9, 6.9 Hz, 3H), 2.99 (dq, J = 6.9, 13.3 Hz, 1H), 3.21 (dq, J = 6.9, 13.3, 1H), 3.77 (s, 3H), 5.39 (s, 1H), 6.84–6.87 (m, 2H), 7.05–7.07 (m, 2H), 7.38–7.40 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.1, 43.5, 55.4, 61.2, 114.4, 117.0, 123.7, 127.5, 128.7, 128.7, 134.0, 140.4, 156.1; IR (KBr) 2983, 2831, 2228, 1880, 1615, 1512, 1448, 1254, 1225, 1035 cm−1; HRMS (EI) calcd for C17H18N2O (M)+ 266.1419, found 266.1423.
2-[(4-Methoxyphenyl)propylamino]-2-phenylacetonitrile 2c
Yield 77% (43.2 mg); yellow oil; Rf = 0.60 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.79 (dd, J = 7.3, 7.3 Hz, 3H), 1.31–1.48 (m, 2H), 2.83 (dt, J = 8.3, 12.8 Hz, 1H), 3.15 (dt, J = 8.3, 12.8 Hz, 1H), 3.78 (s, 3H), 5.37 (s, 1H), 6.83–6.87 (m, 2H), 7.06–7.10 (m, 2H), 7.35–7.38 (m, 3H), 7.40–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 11.4, 20.7, 50.9, 55.4, 61.8, 114.5, 117.0, 124.0, 127.7, 128.7, 128.8, 134.1, 140.8, 156.2; IR (neat) 2962, 2934, 2836, 2228, 1509, 1451, 1246, 1181, 1037, 825 cm−1; HRMS (EI) calcd for C18H20N2O (M)+ 280.1576, found 280.1574.
1); 1H NMR (400 MHz, CDCl3) δ 0.79 (dd, J = 7.3, 7.3 Hz, 3H), 1.31–1.48 (m, 2H), 2.83 (dt, J = 8.3, 12.8 Hz, 1H), 3.15 (dt, J = 8.3, 12.8 Hz, 1H), 3.78 (s, 3H), 5.37 (s, 1H), 6.83–6.87 (m, 2H), 7.06–7.10 (m, 2H), 7.35–7.38 (m, 3H), 7.40–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 11.4, 20.7, 50.9, 55.4, 61.8, 114.5, 117.0, 124.0, 127.7, 128.7, 128.8, 134.1, 140.8, 156.2; IR (neat) 2962, 2934, 2836, 2228, 1509, 1451, 1246, 1181, 1037, 825 cm−1; HRMS (EI) calcd for C18H20N2O (M)+ 280.1576, found 280.1574.
2-[Butyl(4-methoxyphenyl)amino]-2-phenylacetonitrile 2d
Yield 77% (45.1 mg); yellow oil; Rf = 0.10 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 50
ethyl acetate = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.76 (t, J = 7.3 Hz, 3H), 1.16–1.40 (m, 4H), 2.88 (dt, J = 7.3, 13.2 Hz, 1H), 3.16 (dt, J = 7.3, 13.2 Hz, 1H), 3.78 (s, 3H), 5.36 (s, 1H), 6.83–6.87 (m, 2H), 7.05–7.10 (m, 2H), 7.35–7.42 (m, 3H), 7.53–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 20.0, 29.6, 48.8, 55.4, 61.9, 114.4, 116.9, 124.0, 127.7, 128.7, 128.8, 134.0, 140.8, 156.2; IR (neat) 2957, 2933, 2868, 2228, 1510, 1451, 1246, 1039, 825, 696 cm−1; HRMS (EI) calcd for C19H22N2O (M)+ 294.1732, found 294.1726.
1); 1H NMR (400 MHz, CDCl3) δ 0.76 (t, J = 7.3 Hz, 3H), 1.16–1.40 (m, 4H), 2.88 (dt, J = 7.3, 13.2 Hz, 1H), 3.16 (dt, J = 7.3, 13.2 Hz, 1H), 3.78 (s, 3H), 5.36 (s, 1H), 6.83–6.87 (m, 2H), 7.05–7.10 (m, 2H), 7.35–7.42 (m, 3H), 7.53–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.7, 20.0, 29.6, 48.8, 55.4, 61.9, 114.4, 116.9, 124.0, 127.7, 128.7, 128.8, 134.0, 140.8, 156.2; IR (neat) 2957, 2933, 2868, 2228, 1510, 1451, 1246, 1039, 825, 696 cm−1; HRMS (EI) calcd for C19H22N2O (M)+ 294.1732, found 294.1726.
2-[(4-Methoxyphenyl)octylamino]-2-phenylacetonitrile 2e
Yield 70% (48.9 mg); yellow oil; Rf = 0.48 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.85 (t, J = 7.3 Hz, 3H), 1.11–1.41 (m, 12H), 2.86 (dt, J = 8.2, 13.3 Hz, 1H), 3.15 (dt, J = 8.2, 13.3, 1H), 3.78 (s, 3H), 5.37 (s, 1H), 6.83–6.87 (m, 2H), 7.05–7.09 (m, 2H), 7.35–7.43 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 14.1, 22.6, 26.8, 27.4, 29.1, 29.2, 31.7, 49.0, 55.4, 61.8, 114.4, 116.9, 124.0, 127.7, 128.7, 128.8, 134.1, 140.8, 156.2; IR (neat) 2927, 2854, 2229, 1510, 1455, 1244, 1182, 1037, 825, 695 cm−1; HRMS (EI) calcd for C23H30N2O (M)+ 350.2358, found 350.2344.
1); 1H NMR (400 MHz, CDCl3) δ 0.85 (t, J = 7.3 Hz, 3H), 1.11–1.41 (m, 12H), 2.86 (dt, J = 8.2, 13.3 Hz, 1H), 3.15 (dt, J = 8.2, 13.3, 1H), 3.78 (s, 3H), 5.37 (s, 1H), 6.83–6.87 (m, 2H), 7.05–7.09 (m, 2H), 7.35–7.43 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 14.1, 22.6, 26.8, 27.4, 29.1, 29.2, 31.7, 49.0, 55.4, 61.8, 114.4, 116.9, 124.0, 127.7, 128.7, 128.8, 134.1, 140.8, 156.2; IR (neat) 2927, 2854, 2229, 1510, 1455, 1244, 1182, 1037, 825, 695 cm−1; HRMS (EI) calcd for C23H30N2O (M)+ 350.2358, found 350.2344.
2-[Isobutyl(4-methoxyphenyl)amino]-2-phenylacetonitrile 2f
Yield 42% (24.9 mg); yellow oil; Rf = 0.30 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 15
ethyl acetate = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.76 (d, J = 6.9 Hz, 3H), 0.82 (d, J = 6.9 Hz, 3H), 1.56–1.66 (m, 1H), 2.48 (dd, J = 9.2, 12.4 Hz, 1H), 3.05 (dd, J = 9.2, 12.4 Hz, 1H), 3.78 (s, 3H), 5.32 (s, 1H), 6.79–6.87 (m, 2H), 7.10–7.14 (m, 2H), 7.35–7.42 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.2, 20.5, 25.9, 55.4, 57.0, 62.9, 114.4, 116.8, 125.0, 127.9, 128.6, 128.8, 134.0, 141.0, 156.6; IR (neat) 2956, 2870, 2229, 1510, 1465, 1248, 1180, 1038, 718, 695 cm−1; HRMS (EI) calcd for C19H22N2O (M)+ 294.1732, found 294.1740.
1); 1H NMR (400 MHz, CDCl3) δ 0.76 (d, J = 6.9 Hz, 3H), 0.82 (d, J = 6.9 Hz, 3H), 1.56–1.66 (m, 1H), 2.48 (dd, J = 9.2, 12.4 Hz, 1H), 3.05 (dd, J = 9.2, 12.4 Hz, 1H), 3.78 (s, 3H), 5.32 (s, 1H), 6.79–6.87 (m, 2H), 7.10–7.14 (m, 2H), 7.35–7.42 (m, 3H), 7.54–7.56 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.2, 20.5, 25.9, 55.4, 57.0, 62.9, 114.4, 116.8, 125.0, 127.9, 128.6, 128.8, 134.0, 141.0, 156.6; IR (neat) 2956, 2870, 2229, 1510, 1465, 1248, 1180, 1038, 718, 695 cm−1; HRMS (EI) calcd for C19H22N2O (M)+ 294.1732, found 294.1740.
2-[Isopropyl(4-methoxyphenyl)amino]-2-phenylacetonitrile 2g
Yield 28% (15.7 mg); yellow oil; Rf = 0.53 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 10
ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.07 (d, J = 6.4 Hz, 3H), 1.29 (d, J = 6.4 Hz, 3H), 3.66 (qq, J = 6.4, 6.4 Hz, 1H), 3.74 (s, 3H), 5.43 (s, 1H), 6.70–6.74 (m, 2H), 6.97–7.01 (m, 2H), 7.26–7.33 (m, 3H), 7.38–7.41 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.7, 22.6, 51.8, 55.3, 56.3, 113.4, 118.7, 127.1, 127.9, 128.4, 128.5, 134.8, 138.7, 156.6; IR (neat) 2970, 2934, 2226, 1606, 1511, 1247, 1155, 880, 787, 648 cm−1; HRMS (EI) calcd for C18H20N2O (M)+ 280.1576, found 280.1563.
1); 1H NMR (400 MHz, CDCl3) δ 1.07 (d, J = 6.4 Hz, 3H), 1.29 (d, J = 6.4 Hz, 3H), 3.66 (qq, J = 6.4, 6.4 Hz, 1H), 3.74 (s, 3H), 5.43 (s, 1H), 6.70–6.74 (m, 2H), 6.97–7.01 (m, 2H), 7.26–7.33 (m, 3H), 7.38–7.41 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.7, 22.6, 51.8, 55.3, 56.3, 113.4, 118.7, 127.1, 127.9, 128.4, 128.5, 134.8, 138.7, 156.6; IR (neat) 2970, 2934, 2226, 1606, 1511, 1247, 1155, 880, 787, 648 cm−1; HRMS (EI) calcd for C18H20N2O (M)+ 280.1576, found 280.1563.
2-[Cyclohexyl(4-methoxyphenyl)amino]-2-phenylacetonitrile 2h
Yield 24% (15.2 mg); yellow oil; Rf = 0.48 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 10
ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.11–1.43 (m, 5H), 1.58–1.88 (m, 4H), 2.17–2.19 (m, 1H), 3.23–3.29 (m, 1H), 3.73 (s, 3H), 5.47 (s, 1H), 6.68–6.72 (m, 2H), 6.95–6.99 (m, 2H), 7.24–7.31 (m, 3H), 7.34–7.38 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 25.3, 25.8, 29.7, 31.1, 32.6, 55.3, 56.0, 60.0, 113.7, 118.8, 127.5, 127.9, 128.3, 128.4, 134.8, 138.7, 156.7; IR (neat) 2931, 2855, 2227, 1606, 1511, 1452, 1291, 1181, 1037, 696 cm−1; HRMS (EI) calcd for C21H24N2O (M)+ 320.1889, found 320.1879.
1); 1H NMR (400 MHz, CDCl3) δ 1.11–1.43 (m, 5H), 1.58–1.88 (m, 4H), 2.17–2.19 (m, 1H), 3.23–3.29 (m, 1H), 3.73 (s, 3H), 5.47 (s, 1H), 6.68–6.72 (m, 2H), 6.95–6.99 (m, 2H), 7.24–7.31 (m, 3H), 7.34–7.38 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 25.3, 25.8, 29.7, 31.1, 32.6, 55.3, 56.0, 60.0, 113.7, 118.8, 127.5, 127.9, 128.3, 128.4, 134.8, 138.7, 156.7; IR (neat) 2931, 2855, 2227, 1606, 1511, 1452, 1291, 1181, 1037, 696 cm−1; HRMS (EI) calcd for C21H24N2O (M)+ 320.1889, found 320.1879.
2-(4-Bromophenyl)-2-[ethyl(4-methoxyphenyl)amino]acetonitrile 2k
Yield 67% (46.0 mg); yellow oil; Rf = 0.64 (toluene); 1H NMR (400 MHz, CDCl3) δ 1.00 (dd, J = 6.9, 6.9 Hz, 3H), 2.95 (dq, J = 6.9, 10.4 Hz, 1H), 3.20 (dq, J = 6.9, 10.4 Hz, 1H), 3.78 (s, 3H), 5.30 (s, 1H), 6.83–6.87 (m, 2H), 7.03–7.07 (m, 2H), 7.41–7.43 (m, 2H), 7.51–7.54 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.2, 43.9, 55.4, 60.9, 114.5, 116.6, 122.9, 124.2, 129.2, 131.9, 133.2, 140.0, 156.4; IR (neat) 2990, 2954, 2223, 1612, 1516, 1253, 1186, 847, 787, 629 cm−1; HRMS (EI) calcd for C17H17BrN2O (M)+ 344.0524, found 344.0534.2-[Ethyl(4-methoxyphenyl)amino]-2-(4-methoxyphenyl)acetonitrile 2l
Yield 52% (30.7 mg); yellow oil; Rf = 0.32 (toluene); 1H NMR (400 MHz, CDCl3) δ 0.99 (dd, J = 6.9, 6.9 Hz, 3H), 3.00 (dq, J = 7.4, 11.9 Hz, 1H), 3.18 (dq, J = 7.4, 11.9 Hz, 1H), 3.78 (s, 3H), 3.82 (s, 3H), 5.33 (s, 1H), 6.83–6.92 (m, 4H), 7.03–7.07 (m, 2H), 7.42–7.45 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.2, 43.4, 55.3, 55.4, 60.7, 114.0, 114.4, 117.3, 123.9, 126.0, 128.9, 140.4, 156.1, 160.0; IR (neat) 2966, 2938, 2226, 1613, 1514, 1253, 1176, 851, 738, 628 cm−1; HRMS (EI) calcd for C18H20N2O2 (M)+ 296.1525, found 296.1520.2-(2-Bromophenyl)-2-[ethyl(4-methoxyphenyl)amino]acetonitrile 2m
Yield 85% (59.0 mg); yellow oil; Rf = 0.62 (toluene); 1H NMR (400 MHz, CDCl3) δ 0.99 (dd, J = 6.9, 6.9 Hz, 3H), 3.18 (dq, J = 8.2, 11.0 Hz, 2H), 3.77 (s, 3H), 5.57 (s, 3H), 6.78–6.82 (m, 2H), 7.01–7.06 (m, 2H), 7.21–7.29 (m, 2H), 7.39–7.41 (m, 1H), 7.62–7.64 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 13.2, 45.2, 55.3, 60.4, 114.1, 117.0, 124.5, 125.1, 127.3, 130.5, 130.8, 133.2, 133.6, 139.5, 156.6; IR (neat) 2974, 2934, 2228, 1606, 1510, 1247, 1181, 839, 759, 626 cm−1; HRMS (EI) calcd for C17H17BrN2O (M)+ 344.0524, found 344.0528.2-[Ethyl(4-methoxyphenyl)amino]-2-(pyridin-2-yl)acetonitrile 2n
Yield 54% (28.7 mg); yellow oil; Rf = 0.08 (toluene); 1H NMR (400 MHz, CDCl3) δ 1.06 (dd, J = 6.7, 6.7 Hz, 3H), 3.11 (dq, J = 6.7, 10.0 Hz, 1H), 3.33 (dq, J = 6.7, 10.0 Hz, 1H), 3.77 (s, 3H), 5.50 (s, 1H), 6.82–6.85 (m, 2H), 7.04–7.08 (m, 2H), 7.28–7.30 (m, 1H), 7.52–7.54 (m, 1H), 7.69–7.74 (m, 1H), 8.65–8.66 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 13.2, 44.9, 55.4, 63.2, 114.5, 116.7, 122.1, 123.5, 123.5, 137.0, 140.2, 149.6, 154.0, 156.1; IR (neat) 2973, 2935, 2229, 1608, 1512, 1247, 1180, 750, 735, 642 cm−1; HRMS (EI) calcd for C16H17N3O (M)+ 267.1372, found 267.1366.2-[Ethyl(4-methoxyphenyl)amino]-2-(furan-2-yl)acetonitrile 2o
Yield 82% (41.9 mg); yellow oil; Rf = 0.24 (toluene); 1H NMR (400 MHz, CDCl3) δ 1.01 (dd, J = 6.9, 6.9 Hz, 3H), 3.16 (dq, J = 6.9, 10.4 Hz, 2H), 3.78 (s, 3H), 5.34 (s, 1H), 6.34–6.37 (m, 1H), 6.45–6.46 (m, 1H), 6.82–6.86 (m, 2H), 7.01–7.05 (m, 2H), 7.45–7.45 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 13.2, 44.3, 55.1, 55.4, 110.5, 110.6, 114.3, 115.6, 124.1, 139.7, 143.6, 146.8, 156.4; IR (neat) 2975, 2936, 2234, 1608, 1511, 1248, 1089, 1181, 901, 744 cm−1; HRMS (EI) calcd for C15H16N2O2 (M)+ 256.1212, found 256.1224.2-[Ethyl(4-methoxyphenyl)amino]-2-(thiphen-2-yl)acetonitrile 2p
Yield 57% (30.8 mg); yellow oil; Rf = 0.42 (toluene); 1H NMR (500 MHz, CDCl3) δ 1.01 (dd, J = 6.7, 6.7 Hz, 3H), 3.05 (dq, J = 7.3, 10.8 Hz, 1H), 3.22 (dq, J = 7.3, 10.8 Hz, 1H), 3.79 (s, 3H), 5.45 (s, 1H), 6.85–6.92 (m, 2H), 6.97–6.99 (m, 1H), 7.12–7.15 (m, 2H), 7.22–7.23 (m, 1H), 7.34–7.35 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.1, 43.4, 55.4, 57.9, 114.5, 116.4, 124.5, 126.7, 126.9, 138.7, 140.1, 156.7; IR (neat) 2975, 2935, 2231, 1609, 1510, 1246, 1105, 917, 839, 710 cm−1; HRMS (EI) calcd for C15H16N2OS (M)+ 272.0983, found 272.0976.2-[Ethyl(4-methoxyphenyl)amino]-2-(naphthalen-1-yl)acetonitrile 2q
Yield 72% (45.4 mg); yellow oil; Rf = 0.43 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.94 (dd, J = 6.7, 6.7 Hz, 3H), 3.20 (m, 2H), 3.77 (s, 3H), 6.01 (s, 1H), 6.81–6.84 (m, 2H), 6.91–7.03 (m, 2H), 7.39–7.45 (m, 1H), 7.52–7.57 (m, 2H), 7.70–7.71 (m, 1H), 7.86–7.90 (m, 2H), 8.09–8.10 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.1, 44.6, 55.4, 58.0, 114.4, 117.6, 123.3, 123.5, 124.9, 126.2, 126.9, 127.3, 128.8, 129.1, 130.1, 130.9, 133.8, 140.0, 156.0; IR (neat) 2969, 2931, 2229, 1597, 1514, 1250, 1180, 837, 738, 630 cm−1; HRMS (EI) calcd for C21H20N2O (M)+ 316.1576, found 316.1571.2-[Ethyl(4-methoxyphenyl)amino]-2-(naphthalen-2-yl)acetonitrile 2r
Yield 78% (49.6 mg); yellow oil; Rf = 0.31 (toluene); 1H NMR (500 MHz, CDCl3) δ 1.00 (dd, J = 7.3, 7.3 Hz, 3H), 3.02 (dq, J = 7.3, 11.0 Hz, 1H), 3.24 (dq, J = 7.3, 11.0 Hz, 1H), 3.78 (s, 3H), 5.54 (s, 1H), 6.84–6.88 (m, 2H), 7.10–7.14 (m, 2H), 7.51–7.55 (m, 2H), 7.63–7.65 (m, 1H), 7.85–7.88 (m, 3H), 8.03 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.2, 43.5, 55.4, 61.4, 114.5, 117.1, 123.7, 125.0, 126.7, 126.8, 126.9, 127.7, 128.2, 128.7, 131.5, 133.0, 133.3, 140.6, 156.2; IR (neat) 2984, 2973, 2229, 1510, 1249, 1178, 1105, 864, 728, 643 cm−1; HRMS (EI) calcd for C21H20N2O (M)+ 316.1576, found 316.1579.2-Cyclohexyl-2-[ethyl(4-methoxyphenyl)amino]acetonitrile 2s
Yield 31% (16.9 mg); yellow oil; Rf = 0.31 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.89–1.02 (m, 2H), 1.05 (dd, J = 7.3, 7.3 Hz, 3H), 1.12–1.36 (m, 3H), 1.69–1.80 (m, 4H), 2.02–2.04 (m, 1H), 2.15–2.17 (m, 1H), 3.09 (dq, J = 7.3, 11.0 Hz, 1H), 3.19 (dq, J = 7.3, 11.0 Hz, 1H), 3.68 (d, J = 10.4 Hz, 1H), 3.79 (s, 3H), 6.84–6.87 (m, 2H), 7.04–7.07 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 13.4, 25.4, 25.6, 26.3, 30.2, 30.4, 38.7, 44.2, 55.5, 63.1, 114.4, 118.2, 123.8, 141.4, 155.9; IR (neat) 2931, 2853, 2224, 1511, 1245, 1140, 894, 737, 626, 543 cm−1; HRMS (EI) calcd for C17H24N2O (M)+ 272.1889, found 272.1885.Synthesis of 2-[ethyl(4-methoxyphenyl)amino]-2-phenylmalononitrile: general procedure for the synthesis of α-aminomalononitrile 5a
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed (Z)-N-(4-methoxyphenyl)benzimidoylcyanide (0.25 mL, 0.24 mmol, 0.95 M in Et2O) in toluene (2.0 mL) at −40 °C. To it was added EtMgBr (0.20 mL, 0.23 mmol, 0.99 M THF). After the mixture was stirred for 15 min at room temperature,Me2SiCl2 (0.01 mL, 0.12 mmol) was added at 0 °C, and the mixture was stirred at 0 °C for another 15 min. A solution of NIS (40.0 mg, 0.23 mmol) in MeCN (1.5 mL) was added, and the reaction mixture was stirred for 1 min at room temperature. Then, nBu3SnCN (75.9 mg, 0.24 mmol) was added and the mixture was stirred at room temperature for 3 hr. The reaction was quenched with sat aq NaHCO3 (5.0 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nhexane = 15
nhexane = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 5% Et3N) to give the title compound 5a.
1 + 5% Et3N) to give the title compound 5a.
Yield 72% (41.9 mg); yellow oil; Rf = 0.46 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 7.3 Hz, 3H), 3.12 (q, J = 7.3 Hz, 2H), 3.77 (s, 3H), 6.79–6.83 (m, 2H), 7.23–7.26 (m, 2H), 7.41–7.44 (m, 3H), 7.66–7.71 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.3, 45.5, 55.3, 64.7, 113.7, 114.3, 127.4, 129.2, 129.3, 130.6, 132.5, 135.8, 159.0; IR (neat) 2976, 2935, 2362, 2251, 1581, 1507, 1253, 1253, 1171, 1038, 696 cm−1; HRMS (EI) calcd for C18H17N3O (M)+ 291.1372, found 291.1374.
1); 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 7.3 Hz, 3H), 3.12 (q, J = 7.3 Hz, 2H), 3.77 (s, 3H), 6.79–6.83 (m, 2H), 7.23–7.26 (m, 2H), 7.41–7.44 (m, 3H), 7.66–7.71 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.3, 45.5, 55.3, 64.7, 113.7, 114.3, 127.4, 129.2, 129.3, 130.6, 132.5, 135.8, 159.0; IR (neat) 2976, 2935, 2362, 2251, 1581, 1507, 1253, 1253, 1171, 1038, 696 cm−1; HRMS (EI) calcd for C18H17N3O (M)+ 291.1372, found 291.1374.
2-(4-Bromophenyl)-2-[ethyl(4-methoxyphenyl)amino]malononitrile 5b
Yield 59% (43.8 mg); yellow oil; Rf = 0.76 (toluene); 1H NMR (400 MHz, CDCl3) δ 0.90 (t, J = 6.9 Hz, 3H), 3.13 (q, J = 6.9 Hz, 2H), 3.78 (s, 3H), 6.80–6.84 (m, 2H), 7.19–7.23 (m, 2H), 7.52–7.58 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 13.3, 45.7, 55.3, 64.1, 113.3, 114.4, 125.2, 129.0, 129.2, 131.6, 132.5, 135.4, 159.0; IR (neat) 2979, 2936, 2231, 1607, 1510, 1251, 1181, 832, 739, 628 cm−1; HRMS (EI) calcd for C18H16BrN3O (M)+ 369.0477, found 369.0465.2-[Ethyl(4-methoxyphenyl)amino]-2-(4-methoxyphenyl)malononitrile 5d
Yield 27% (17.5 mg); yellow oil; Rf = 0.46 (toluene); 1H NMR (400 MHz, CDCl3) δ 0.89 (t, J = 7.3 Hz, 3H), 3.12 (q, J = 7.3 Hz, 2H), 3.79 (s, 3H), 6.80–6.84 (m, 2H), 6.89–6.93 (m, 2H), 7.20–7.24 (m, 2H), 7.56–7.60 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.4, 45.4, 55.3, 55.5, 64.2, 113.9, 114.3, 114.5, 124.3, 128.9, 129.3, 135.9, 158.9, 161.1; IR (neat) 2981, 2940, 2247, 1605, 1512, 1285, 1118, 974, 758, 633 cm−1; HRMS (EI) calcd for C19H19N3O2 (M)+ 321.1477, found 326.1468.2-(2-Bromophenyl)-2-[ethyl(4-methoxyphenyl)amino]malononitrile 5c
Yield 61% (45.1 mg); m.p. = 68–71 °C; yellow solid; Rf = 0.57 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.94 (t, J = 6.7 Hz, 3H), 3.15 (q, J = 7.3 Hz, 2H), 3.77 (s, 3H), 6.79–6.82 (m, 2H), 7.29–7.39 (m, 4H), 7.67–7.75 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 13.2, 45.5, 55.3, 65.7, 112.5, 114.2, 122.4, 127.9, 128.9, 129.7, 130.8, 131.9, 135.7, 136.2, 158.9; IR (KBr) 2978, 2933, 2227, 1606, 1510, 1252, 1109, 844, 752, 629 cm−1; HRMS (EI) calcd for C18H16BrN3O (M)+ 369.0477, found 369.0494.2-[Ethyl(4-methoxyphenyl)amino]-2-(pyridine-2-yl)malononitrile 5e
Yield 45% (26.5 mg); yellow oil; Rf = 0.11 (toluene); 1H NMR (400 MHz, CDCl3) δ 0.94 (t, J = 6.9 Hz, 3H), 3.20 (q, J = 6.9 Hz, 2H), 3.77 (s, 3H), 6.79–6.82 (m, 2H), 7.22–7.27 (m, 2H), 7.35–7.39 (m, 1H), 7.72–7.74 (m, 1H), 7.78–7.82 (m, 1H), 8.63–8.65 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 13.3, 46.2, 55.3, 67.0, 113.2, 114.3, 122.3, 125.1, 129.2, 135.6, 137.9, 149.6, 151.7, 158.9; IR (neat) 2973, 2938, 2250, 1610, 1509, 1232, 1157, 965, 850, 634 cm−1; HRMS (EI) calcd for C17H16N4O (M)+ 292.1324, found 292.1319.2-[Ethyl(4-methoxyphenyl)amino]-2-(furan-2-yl)malononitrile 5f
Yield 8% (4.3 mg); yellow oil; Rf = 0.50 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.96 (t, J = 7.3 Hz, 3H), 3.24 (q, J = 6.7 Hz, 2H), 3.78 (s, 3H), 6.35–6.36 (m, 1H), 6.56–6.57 (m, 1H), 6.80–6.83 (m, 2H), 7.16–7.19 (m, 2H), 7.50–7.50 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.3, 45.8, 55.3, 59.0, 111.2, 111.8, 113.2, 114.3, 128.9, 135.2, 142.6, 145.2, 159.0; IR (neat) 2979, 2932, 2202, 1509, 1246, 1168, 975, 758, 650, 593 cm−1; HRMS (EI) calcd for C16H15N3O2 (M)+ 281.1164, found 281.1170.2-[Ethyl(4-methoxyphenyl)amino]-2-(thiophen-2-yl)malononitrile 5g
Yield 29% (17.4 mg); yellow oil; Rf = 0.47 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.93 (t, J = 7.3 Hz, 3H), 3.12 (q, J = 7.3 Hz, 2H), 3.81 (s, 3H), 6.88–6.89 (m, 2H), 6.99–7.00 (m, 1H), 7.34–7.36 (m, 2H), 7.39–7.40 (m, 1H), 7.47–7.48 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.2, 45.4, 55.4, 60.6, 113.0, 114.5, 126.9, 129.2, 129.4, 129.6, 136.1, 136.5, 159.3; IR (neat) 2978, 2936, 2231, 1607, 1510, 1242, 1033, 844, 715, 761 cm−1; HRMS (EI) calcd for C16H15N3OS (M)+ 297.0936, found 297.0929.2-[Ethyl(4-methoxyphenyl)amino]-2-(maphthalen-1-yl)malononitrile 5h
Yield 39% (26.9 mg); yellow oil; Rf = 0.50 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.91 (t, J = 7.3 Hz, 3H), 3.28 (q, J = 7.3 Hz, 2H), 3.73 (s, 3H), 6.72–6.74 (m, 2H), 7.22–7.26 (m, 2H), 7.34–7.42 (m, 1H), 7.60–7.65 (m, 1H), 7.70–7.73 (m, 1H), 7.84–7.92 (m, 3H), 8.94–8.95 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.5, 45.9, 55.3, 65.5, 113.5, 114.2, 124.6, 124.8, 126.2, 126.8, 127.1, 128.0, 128.8, 129.2, 129.4, 132.2, 134.5, 135.6, 158.8; IR (neat) 2977, 2934, 2228, 1606, 1510, 1251, 1111, 843, 758, 652 cm−1; HRMS (EI) calcd for C22H19N3O (M)+ 341.1528, found 341.1525.2-[Ethyl(4-methoxyphenyl)amino]-2-(maphthalen-2-yl)malononitrile 5i
Yield 52% (35.2 mg); yellow oil; Rf = 0.47 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.92 (t, J = 7.3 Hz, 3H), 3.12 (q, 6.7 Hz, 2H), 3.75 (s, 3H), 6.81–6.84 (m, 2H), 7.27–7.35 (m, 2H), 7.55–7.59 (m, 2H), 7.80–7.97 (m, 4H), 8.15–8.15 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 13.3, 45.3, 55.3, 64.9, 113.7, 114.4, 123.4, 127.2, 127.6, 127.7, 127.9, 128.6, 129.3, 129.7, 129.7, 132.6, 133.9, 136.0, 159.0; IR (neat) 2979, 2936, 2231, 1606, 1582, 1251, 1034, 811, 758, 656 cm−1; HRMS (EI) calcd for C22H19N3O (M)+ 341.1528, found 341.1538.2-[Butyl(4-methoxyphenyl)amino]-2-phenylmalononitrile 5j
Yield 32% (20.6 mg); yellow oil; Rf = 0.59 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.75 (t, J = 7.3 Hz, 3H), 1.20–1.26 (m, 4H), 3.06 (t, J = 6.1 Hz, 2H), 3.78 (s, 3H), 6.80–6.83 (m, 2H), 7.22–7.26 (m, 2H), 7.40–7.44 (m, 3H), 7.66–7.70 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 13.6, 19.7, 29.6, 50.5, 55.3, 64.9, 113.7, 114.3, 127.5, 129.2, 130.6, 132.5, 136.1, 158.9; IR (neat) 2959, 2933, 2230, 1607, 1510, 1250, 1181, 823, 747, 633 cm−1; HRMS (EI) calcd for C20H21N3O (M)+ 319.1685, found 319.1687.2-[4-Methoxyphenyl(octyl)amino]-2-phenylmalononitrile 5k
Yield 23% (17.5 mg); yellow oil; Rf = 0.62 (toluene); 1H NMR (500 MHz, CDCl3) δ 0.85 (t, J = 7.3 Hz, 3H), 1.10–1.22 (m, 12H), 3.05 (t, J = 6.1 Hz, 2H), 6.80–6.82 (m, 2H), 7.23–7.24 (m, 2H), 7.43–7.44 (m, 3H), 7.67–7.69 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 14.0, 22.6, 26.5, 27.5, 29.0, 29.1, 31.7, 50.8, 55.3, 113.7, 114.3, 127.5, 129.2, 130.6, 132.5, 136.2, 158.9; IR (neat) 2954, 2855, 2282, 1607, 1510, 1250, 1035, 841, 747, 663 cm−1; HRMS (EI) calcd for C24H29N3O (M)+ 375.2311, found 375.2322.2-[Ethyl(4-methoxyphenyl)amino]-5-oxo-2-phenylhexanenitrile 7
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed a solution of (Z)-N-(4-methoxyphenyl)benzimidoylcyanide (47.3 mg, 0.20 mmol) in toluene (2.0 mL) at −40 °C. To it was added EtMgBr (0.12 mL, 0.24 mmol, 2.02 M in Et2O). After the mixture was stirred for 15 min at room temperature, it was cooled to −40 °C. To the mixture MVK (0.020 mL, 0.24 mmol) was added, and it was stirred at −40 °C for 4 h. The reaction was quenched with sat aq NaHCO3 (5 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by alumina column chromatography (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 5
EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title compound 7.
1) to give the title compound 7.
Yield 50% (33.7 mg); yellow oil; Rf = 0.46 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 5
ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (500 MHz, CDCl3) δ 0.73 (dd, J = 6.7, 6.7 Hz, 3H), 1.84–1.91 [(m, 5H) including a singlet of C(O)CH3 at δ = 1.89], 1.97 (dt, J = 6.7, 11.0 Hz, 1H), 2.36 (dt, J = 6.7, 11.0 Hz, 1H), 2.64 (dq, J = 6.7, 11.0 Hz, 1H), 2.98 (dq, J = 6.7, 11.0 Hz, 1H), 3.82 (s, 3H), 6.87–6.92 (m, 2H), 7.36–7.44 (m, 5H), 7.67–7.70 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 13.6, 29.9, 35.4, 38.9, 46.2, 55.3, 69.9, 114.3, 119.6, 126.5, 128.7, 128.8, 129.9, 137.8, 139.0, 158.4, 206.2; IR (neat) 2973, 2934, 2221, 1718, 1608, 1511, 1244, 1168, 1034, 702 cm−1; HRMS (EI) calcd for C21H24N2O2 (M)+ 336.1838, found 336.1846.
1); 1H NMR (500 MHz, CDCl3) δ 0.73 (dd, J = 6.7, 6.7 Hz, 3H), 1.84–1.91 [(m, 5H) including a singlet of C(O)CH3 at δ = 1.89], 1.97 (dt, J = 6.7, 11.0 Hz, 1H), 2.36 (dt, J = 6.7, 11.0 Hz, 1H), 2.64 (dq, J = 6.7, 11.0 Hz, 1H), 2.98 (dq, J = 6.7, 11.0 Hz, 1H), 3.82 (s, 3H), 6.87–6.92 (m, 2H), 7.36–7.44 (m, 5H), 7.67–7.70 (m, 2H); 13C NMR (125 MHz, CDCl3) δ 13.6, 29.9, 35.4, 38.9, 46.2, 55.3, 69.9, 114.3, 119.6, 126.5, 128.7, 128.8, 129.9, 137.8, 139.0, 158.4, 206.2; IR (neat) 2973, 2934, 2221, 1718, 1608, 1511, 1244, 1168, 1034, 702 cm−1; HRMS (EI) calcd for C21H24N2O2 (M)+ 336.1838, found 336.1846.
2-[Ethyl(4-methoxyphenyl)amino]-3-oxo-2,3-diphenylpropanenitrile 8
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon was placed a solution of (Z)-N-(4-methoxyphenyl)benzimidoylcyanide (47.3 mg, 0.20 mmol) in toluene (2.0 mL) and nhexane (1.0 mL) at −40 °C. To it was added EtMgBr (0.12 mL, 0.24 mmol, 2.02 M in Et2O). After the mixture was stirred for 15 min at room temperature, it was cooled to −40 °C. To the mixture was added benzoyl chloride (0.070 mL, 0.60 mmol), and it was stirred at −40 °C for 35 min. The reaction was quenched with sat aq NaHCO3 (5 mL), and the whole mixture was extracted with ethyl acetate (5 mL × 3). The combined extracts were washed with brine (5 mL × 2), dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 10
EtOAc = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 5% Et3N) to give the title compound 8.
1 + 5% Et3N) to give the title compound 8.
Yield 74% (54.6 mg); yellow oil; Rf = 0.22 (nhexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 6
ethyl acetate = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 1.05 (dd, J = 6.9, 6.9 Hz, 3H), 3.08 (dq, J = 6.9, 12.3 Hz, 1H), 3.27 (dq, J = 6.9, 12.3 Hz, 1H), 3.70 (s, 3H), 6.64–6.66 (m, 2H), 7.18–7.45 (m, 8H), 7.62–7.64 (m, 2H), 7.81–7.83 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.5, 48.5, 55.2, 79.6, 113.6, 118.2, 128.1, 128.2, 128.7, 129.0, 129.3, 129.7, 133.1, 133.6, 134.6, 138.0, 157.6, 190.9; IR (neat) 2977, 2222, 1681, 1511, 1245, 1181, 1035, 915, 700, 639 cm−1; HRMS (EI) calcd for C24H22N2O2 (M)+ 370.1681, found 370.1672.
1); 1H NMR (400 MHz, CDCl3) δ 1.05 (dd, J = 6.9, 6.9 Hz, 3H), 3.08 (dq, J = 6.9, 12.3 Hz, 1H), 3.27 (dq, J = 6.9, 12.3 Hz, 1H), 3.70 (s, 3H), 6.64–6.66 (m, 2H), 7.18–7.45 (m, 8H), 7.62–7.64 (m, 2H), 7.81–7.83 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.5, 48.5, 55.2, 79.6, 113.6, 118.2, 128.1, 128.2, 128.7, 129.0, 129.3, 129.7, 133.1, 133.6, 134.6, 138.0, 157.6, 190.9; IR (neat) 2977, 2222, 1681, 1511, 1245, 1181, 1035, 915, 700, 639 cm−1; HRMS (EI) calcd for C24H22N2O2 (M)+ 370.1681, found 370.1672.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) and Innovative Areas “Organic Synthesis Based on Reaction Integration. Development of New Methods and Creation of New Substances” from JSPS and MEXT.Notes and references
- For C-alkylation to α-iminoesters, see: (a) J.-C. Fiaud and H. B. Kagan, Tetrahedron Lett., 1970, 21, 1813–1816 CrossRef; (b) L. M. Harwood, K. J. Vines and M. G. B. Drew, Synlett, 1996, 1051–1053 CAS; (c) D. Ferraris, B. Young, T. Dudding and T. Lectka, J. Am. Chem. Soc., 1998, 120, 4548–4549 CrossRef CAS; (d) A. Córdova, W. Notz, G. Zhong, J. M. Betancort and C. F. Barbas, J. Am. Chem. Soc., 2002, 124, 1842–1843 CrossRef PubMed; (e) K. P. Chiev, S. Roland and P. Mangeney, Tetrahedron: Asymmetry, 2002, 13, 2205–2209 CrossRef CAS; (f) P. Wipf and C. R. J. Stephenson, Org. Lett., 2003, 5, 2449–2452 CrossRef CAS PubMed; (g) M. Mitani, Y. Tanaka, A. Sawada, A. Misu and Y. Matsumoto, Eur. J. Org. Chem., 2008, 1383–1391 CrossRef CAS; (h) P. Fu, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2008, 130, 5530–5541 CrossRef CAS PubMed; (i) S. Fustero, N. Mateu, L. Albert and J. L. Aceña, J. Org. Chem., 2009, 74, 4429–4432 CrossRef CAS PubMed; (j) K. Yeung, F. Talbot, G. P. Howell, A. P. Pulis and D. J. Procter, ACS Catal., 2019, 9, 1655–1661 CrossRef CAS.
- For N-alkylation to α-iminoesters, see: (a) J.-C. Fiaud and H. B. Kagan, Tetrahedron Lett., 1971, 12, 1019–1022 CrossRef; (b) Y. Yamamoto and W. Ito, Tetrahedron, 1988, 44, 5415–5423 CrossRef CAS; (c) K. Uneyama, F. Yan, S. Hirama and T. Katagiri, Tetrahedron Lett., 1996, 37, 2045–2048 CrossRef CAS; (d) S. E. Yoo and Y. D. Gong, Heterocycles, 1997, 45, 1251–1255 CrossRef CAS; (e) M. P. Bertrand, L. Feray, R. Nouguier and P. Perfetti, Synlett, 1999, 1148–1150 CrossRef CAS; (f) M. Mae, H. Amii and K. Uneyama, Tetrahedron Lett., 2000, 41, 7893–7896 CrossRef CAS; (g) K. P. Chiev, S. Roland and P. Mangeney, Tetrahedron: Asymmetry, 2002, 13, 2205–2209 CrossRef CAS; (h) J. S. Dickstein, M. W. Fennie, A. L. Norman, B. J. Paulose and M. C. Kozlowski, J. Am. Chem. Soc., 2008, 130, 15794–15795 CrossRef CAS PubMed; (i) J. S. Dickstein and M. C. Kozlowski, Chem. Soc. Rev., 2008, 37, 1166–1173 RSC; (j) J. M. Curto, J. S. Dickstein, S. Berritt and M. C. Kozlowski, Org. Lett., 2014, 16, 1948–1951 CrossRef CAS PubMed.
- For N-alkylation to α-iminoesters found in our laboratories, see: (a) Y. Niwa, K. Takayama and M. Shimizu, Bull. Chem. Soc. Jpn., 2002, 75, 1819–1825 CrossRef CAS; (b) Y. Niwa and M. Shimizu, J. Am. Chem. Soc., 2003, 125, 3720–3721 CrossRef CAS PubMed; (c) M. Shimizu, H. Itou and M. Miura, J. Am. Chem. Soc., 2005, 127, 3296–3297 CrossRef CAS PubMed; (d) I. Mizota and M. Shimizu, Chem. Rec., 2016, 16, 688–702 CrossRef CAS PubMed; (e) I. Mizota, Y. Tadano, Y. Nakamura, T. Haramiishi, M. Hotta and M. Shimizu, Org. Lett., 2019, 21, 2663–2667 CrossRef CAS PubMed; (f) M. Shimizu, H. Imazato, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 17341–17346 RSC; (g) M. Shimizu, M. Mushika, I. Mizota and Y. Zhu, RSC Adv., 2019, 9, 23400–23407 RSC.
- M. Shimizu, M. Tateishi and I. Mizota, Chem. Lett., 2014, 43, 1752–1754 CrossRef.
- Hydrolysis: V. Yu, K. Armando and J. L. Pombeiro, Inorg. Chim. Acta, 2005, 358, 1–21 CrossRef , and refernces cited therein.
- Reduction: J. Barrault and Y. Pouilloux, Catal. Today, 1997, 37, 137–153 CrossRef CAS.
- Addition: A. L. Smith and P. Tan, J. Chem. Educ., 2006, 83, 1654–1657 CrossRef CAS.
- Reductive decyanation: (a) C. E. Berkoff, D. E. Rivard, D. Kirkpatrick and J. L. Ives, Synth. Commun., 1980, 10, 939–945 CrossRef CAS; (b) J.-M. Mattalia, C. Marchi-Delapierre, H. Hazimeh and M. Chanon, ARKIVOC, 2006, 90–118 Search PubMed.
- P. Fontaine, A. Chiaroni, G. Masson and J. Zhu, Org. Lett., 2008, 10, 1509–1512 CrossRef CAS.
- (a) N. Picci, M. Pocci, A. Gugliuzza, F. Puoci, A. De Munno, F. Iemma and V. Bertini, Heterocycles, 2001, 55, 2075–2084 CrossRef CAS; (b) J. J. Garcia, N. M. Brunkan and W. D. Jones, J. Am. Chem. Soc., 2002, 124, 9547–9555 CrossRef CAS PubMed; (c) J. M. Penney, Tetrahedron Lett., 2004, 45, 2667–2669 CrossRef CAS; (d) J. M. Penney and J. A. Miller, Tetrahedron Lett., 2004, 45, 4989–4992 CrossRef CAS; (e) Y. Nakao, S. Oda and T. Hiyama, J. Am. Chem. Soc., 2004, 126, 13904–13905 CrossRef CAS; (f) Y. Nakao, A. Yada, S. Ebata and T. Hiyama, J. Am. Chem. Soc., 2007, 129, 2428–2429 CrossRef CAS; (g) M. Tobisu, Y. Kita, Y. Ano and N. Chatani, J. Am. Chem. Soc., 2008, 130, 15982–15989 CrossRef CAS; (h) D.-G. Yu, M. Yu, B.-T. Guan, B.-J. Li, Y. Zheng, Z.-H. Wu and Z.-J. Shi, Org. Lett., 2009, 11, 3374–3377 CrossRef CAS; (i) M. Columbo, S. Vallese, I. Peretto, X. Jacq, J.-C. Rain, F. Colland and P. Guedat, Chem. Med. Chem, 2010, 5, 552–558 CrossRef; (j) Y. Kita, M. Tobisu and N. Chatani, Org. Lett., 2010, 12, 1864–1867 CrossRef CAS; (k) K. Nakai, T. Kurahashi and S. Matsubara, J. Am. Chem. Soc., 2011, 133, 11066–11068 CrossRef CAS; (l) M. Tobisu, H. Kinuta, Y. Kita, E. Rémond and N. Chatani, J. Am. Chem. Soc., 2012, 134, 115–118 CrossRef CAS PubMed; (m) A. D. Thompson and M. P. Huestis, J. Org. Chem., 2013, 78, 762–769 CrossRef CAS PubMed.
- (a) T. Opatz, Synthesis, 2009, 1941–1959 CrossRef CAS; (b) N. Otto and T. Opatz, Chem. – Eur. J., 2014, 20, 13064–13077 CrossRef CAS PubMed; (c) C. Grundke and T. Opatz, Green Chem., 2019, 21, 2362–2366 RSC , and references cited therein.
- Addition of methyl-, benzyl-, and arylmetal reagents to α-iminoesters is available only for the doubly activated derivatives: (a) I. Mizota, C. Ueda, Y. Tesong, Y. Tsujimoto and M. Shimizu, Org. Lett., 2018, 20, 2291–2296 CrossRef CAS . These reactivity differences could be explained in terms of the different pKa values and nucleophilicity of the reagents, see: ; (b) F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456–463 CrossRef CAS; (c) H. Mayr and A. R. Ofial, J. Phys. Org. Chem., 2008, 21, 584–595 CrossRef CAS , and references cited therein. See also, ref. 3a and g.
- R. J. H. Gregory, Chem. Rev., 1999, 99, 3649–3682 CrossRef CAS.
- M. Tanaka, Tetrahedron Lett., 1980, 21, 2959–2962 CrossRef CAS.
- Although we have not fully examined the substituents at the starting imino nitrogen, we presume that a similar reactivity tendency to that observed for previously reported α-iminoesters would be expected for the present α-iminonitriles.
- Aminomalononitriles are useful units for further construction of molecules. See for example: (a) J. P. Ferris, R. A. Sanchez and R. W. Mancuso, Org. Synth., 1973, 5, 32–34 Search PubMed; (b) J. P. Ferris and R. A. Sanchez, Org. Synth., 1973, 5, 344–345 Search PubMed; (c) M. A. Metwally and M. A. Mogieb, Synth. Commun., 2001, 31, 1901–1905 CrossRef CAS; (d) D. Kikelj and U. Urleb, Thiazoles, in Science of Synthesis Houben-Weyl Methods of Organic Chemistry, ed. E. Schaumann, Thieme, Stuttgart, New York, 2002, pp. 627–833 Search PubMed; (e) V. Arava and S. Gogireddy, Der Pharma Chem., 2013, 5, 232–239 CAS; (f) A. W. Schwartz, H. Joosten and A. B. Voet, BioSystems, 1982, 15, 191–193 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nj05114g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2020 |