Hollow multishelled structures revive high energy density batteries
Jiangyan
Wang
ab,
Yi
Cui
cd and
Dan
Wang
 *ab
*ab
aState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: danwang@ipe.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, China
cDepartment of Materials Science and Engineering, Stanford University, Stanford, California 94305, USA
dStanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, California 94025, USA
First published on 9th July 2020
Abstract
Hollow structures have been shown to be fruitful in addressing the cycling-stability problem of high-capacity electrode materials. However, we have noticed that there exist misconceptions toward the energy density of hollow-structured electrodes. In this Focus Article, the indispensability of hollow structures for stable high energy density batteries is discussed. Additionally, the merits of hollow multishelled structures (HoMSs) superior to their single-shelled counterparts mainly including optimizing the volumetric energy density, improving the mechanical robustness and enabling smart safe energy-storage behaviors have also been highlighted. The goal of the current article is to clarify that a HoMS-based electrode is indispensable to realize a practically high energy density in addition to lengthening the cycling lifespan and guide the future development of HoMSs to further improve the performance of rechargeable batteries.
Introduction
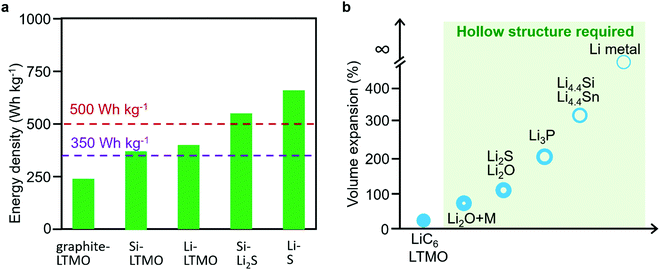
Hollow structures with well-defined boundaries and interior cavities possess many beneficial properties including low density, large specific surface area, short charge and mass transport path, and high volumetric loading capacity.1–6 As a result, hollow structures have been widely explored as a promising functional candidate in energy-related technologies especially in rechargeable batteries such as alkali metal-ion batteries (alkali metals typically include lithium (Li), sodium (Na), potassium (K), etc.), alkali metal batteries, alkali metal–sulfur batteries, and so forth.7–17The state-of-the-art lithium-ion battery (LIB), which is composed of a graphite anode and a Li transition-metal oxide (LTMO)/phosphate cathode, has become the dominant sustainable energy provider for electronics and electrical vehicles. However, the current graphite–LTMO LIB is approaching its theoretical limits with narrow room to meet the worldwide increasing demands for higher energy density of rechargeable batteries. For example, China has proposed a goal of achieving 350 W h kg−1 in 2030 for rechargeable batteries,18 while the US Department of Energy put forward a “Battery 500” program which aims to realize an even higher energy density of 500 W h kg−1 in the next few decades.19 As shown in Fig. 1a, replacing the graphite anode with silicon (Si) or Li metal could double the energy density and theoretically achieve an energy density of 350 W h kg−1, and by further replacing the LTMO cathode with sulfur (S) or lithium sulfide, the energy density could be further pushed to 500 W h kg−1. Unfortunately, high-capacity electrode materials always suffer from a large volume change during the lithiation/delithiation process (Fig. 1b), which results in a broken electrode structure, unstable and accumulated solid–electrolyte interphase (SEI) formation, and thus poor Coulombic efficiencies (CEs) and short cycling life.
Over the past decade, hollow structures have been fruitful in addressing the above-mentioned failure model. With sufficient inner void to buffer the destructive volume expansion and alleviate stress during lithiation, hollow structured electrodes can maintain good structural integrity and achieve much better cycling stability than their solid counterparts. However, concerns regarding the fact that the inner void of hollow structured electrodes would sacrifice the energy density of batteries have drawn considerable attention. Here we would like to clarify that hollow structures especially hollow multishelled structures (HoMSs) are indispensable to achieve a practically high energy density for next-generation rechargeable batteries.
Hollow structures are indispensable for stable high energy density batteries
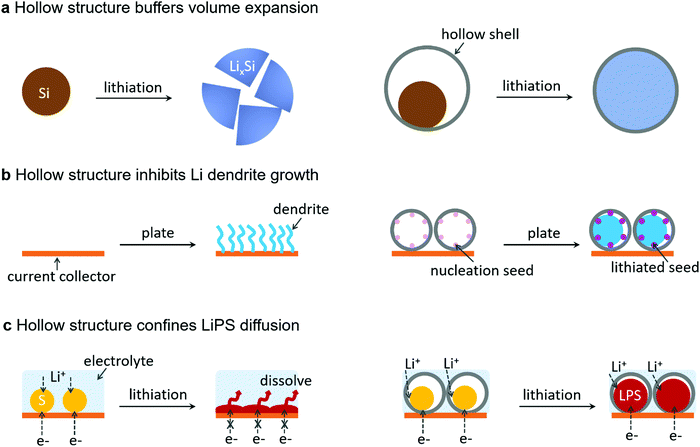
Although lots of literature studies have reported the adoption of hollow structures to improve the cycling stability of rechargeable batteries, some misconceptions exist in the literature that the inner cavity of the hollow structure would decrease the total energy density of batteries. In the following, we would like to point out that instead of lowering the energy density of batteries, hollow structures are indispensable to realize a practically high energy density given the following aspects (Fig. 2).(1) Hollow structures with a pre-reserved inner cavity could accommodate the large volume expansion of high-capacity electrode materials.
(2) Hollow structures as the host for the alkali metal anode could inhibit metal dendrite growth and limit metal–electrolyte contact area, thus achieving high CEs, better safety and stability.
(3) Hollow structures as a sulfur carrier/lithium polysulfide (LPS) reservoir could avoid the electron-blocking effect ascribed to the covering of LPSs on the current collector and simultaneously inhibit the diffusion of soluble long-chain LPSs into the electrolyte, thus enabling high sulfur utilization and CEs, and better cycling stability.
The fast development of electronics and electrical vehicles relies on next-generation rechargeable batteries with higher energy density and lower cost. Si, with a theoretical capacity (3579 mA h g−1) 10 times as high as that of the current commercial graphite anode, has been widely considered as a promising anode material for next-generation LIBs. However, its large volume expansion during lithiation (∼300% for Li15Si4) induces structural breakdown and an unstable SEI, thus leading to a short cycling life and low CEs.20,21 To address this problem, the first trial is decreasing the size to the nanoscale. Cui's group demonstrated the first breakthrough by using Si nanowires as anode materials,22 and following this concept, other nanostructured Si anodes including Si nanoparticles23 and amorphous-crystalline Si core–shell nanowires24 have also been reported. Although these nanostructures have shown much better resistance to pulverization, the SEI remained unstable and continuously accumulated due to their outward expansion during lithiation. To stabilize the SEI, hollow structures including double-walled Si–SiO2 hollow tubes,25 nanosized Si-amorphous C yolk–shell26 and microsized Si–graphene yolk–shell27 have been investigated. With the interior void to buffer the volume expansion and the outer mechanically strong shell to limit Si expansion inward, both the whole structural integrity and the SEI remained stable during repeated cycles (Fig. 2a).
The Li metal has been considered as the ultimate anode of choice due to its lowest electrochemical potential (−3.04 vs. the standard hydrogen electrode, SHE) and highest theoretical capacity (3862 mA h g−1). However, the Li metal anode suffers from some severe challenges, such as high reactivity, dendritic growth, huge volume change during cycling, etc. The high reactivity leads to vigorous side reactions between the Li metal and electrolyte, while its dendritic growth and huge volume change crack the SEI layer, inducing repeated SEI breakdown/formation which severely shortens the cycle lifespan. Moreover, its dendritic growth could cause internal short circuit and bring about battery safety concerns.28 Among various efforts to resolve these problems, the adoption of a hollow structure for the Li metal anode has achieved noticeable progress. One method29 is to cover the surface of the current collector with a carbon hollow sphere, which is lifted up after Li plating from underneath due to the weak binding between the carbon hollow sphere and the current collector. Benefiting from the decreased Li–electrolyte contact area and inhibited Li dendrite growth, the cycling stability was enhanced. Another effective method15,30 is to embed nucleation seeds (such as Au, ZnO, Si, etc.) inside mechanical hollow capsules; the nucleation seed could guide the Li metal to plate inside the hollow capsules while the strong carbon shell stops the electrolyte from entering inside, thus hindering Li dendrite growth as well as stabilizing the SEI (Fig. 2b). As a result, high CEs (>98%) could be maintained after hundreds of cycles even in carbonate electrolytes.
In addition to anodes, a hollow structure is also indispensable for high-capacity cathode materials. Sulfur has a 5 times higher theoretical capacity (1680 mA h g−1) than conventional LTMO cathode materials. However, the practically achievable capacity is much lower than the theoretical value, which is mainly because of its large volume expansion (80%), insulating nature of both sulfur and its lithiation products, and the shuttle effect caused by the soluble long-chain LPSs. Tremendous efforts have been devoted to solving these problems. Among them, a hollow structure as a sulfur carrier/lithium polysulfide (LiPS) reservoir has shown great promise. Since the first work of adopting carbon nanotubes as a sulfur host,31 various conductive carbon hollow structures have been investigated for sulfur cathodes.32,33 The conductive carbon host can improve the conductivity of sulfur and avoid the electron-blocking effect ascribed to the covering of its lithiation products on the current collector; besides, the carbon host can physically inhibit the diffusion of soluble LPSs into the electrolyte (Fig. 2c), thus enabling higher sulfur utilization and better cycling life. Despite the progress, the weak binding between the nonpolar carbon host and polar LPSs could cause severe detachment of active materials after several cycles. Therefore, polar hosts including N-doped carbon, metal oxide/sulfide/nitride, etc. have been developed. Typically, single-shelled and multi-shelled TiO2−x hollow spheres have been adopted to both physically confine and chemically anchor LPSs to inhibit the shuttle effect, achieving significantly enhanced cycling performance.34,35
HoMSs are more promising to revive high energy density batteries than their single-shelled counterparts
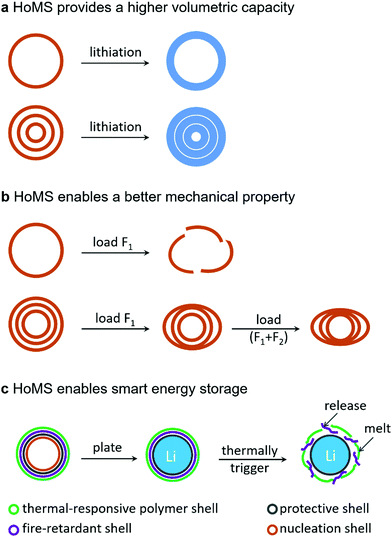
It's worth noting that, compared to a single-shelled hollow structure, a hollow multishelled structure is more promising as the electrode of rechargeable batteries given the following advantages (Fig. 3):(1) The HoMS-based electrode can provide a higher volumetric capacity than its single-shelled counterpart.
(2) The HoMS-based electrode enables better mechanical properties than its single-shelled counterpart.
(3) The heterogeneous HoMS-based electrode enables thermal-responsive fire-retardant properties for smart safe lithium batteries.
As discussed above, the inner void space of a hollow structure could buffer the volume expansion of electrode materials during lithiation, thus maintaining good cycling stability. However an overlarge void ratio (the content of interior void volume in the whole volume of both solid shell and interior void space) would induce a small volumetric capacity, while a too small void ratio could not accommodate the volume expansion thus leading to structural breakdown; only a suitable void ratio compatible with the volume expansion could guarantee a good cycling stability and simultaneously achieve a high volumetric capacity. Compared to a single-shelled hollow structure, a HoMS possesses much more abundant geometrical diversity. As a result, HoMSs offer a great chance to control the void ratio by adjusting the shell number, inter-shell gap and shell thickness. Wang's group reported a general sequential templating approach for the synthesis of HoMSs. By controlling the synthesis condition, both the geometrical and compositional characteristics of HoMSs could be accurately controlled to optimize the electrochemical performance.36–38 As a typical example, Co3O4 HoMSs as anodes for LIBs exhibit differential performance with different shell numbers:39 the single-shelled structure achieved good cycling stability yet suffered from a low volumetric capacity due to the overlarge void ratio, while the quadruple-shelled structure exhibited poor cycling stability due to a smaller void ratio than the volume expansion of Co3O4 which caused structure cracking. Comparatively, the triple-shelled structure with a suitable void ratio achieved not only a good cycling life but also a good practical volumetric capacity (Fig. 3a).
Another key parameter for electrode materials is their mechanical property. During the practical electrode film fabrication, a calendering process is adopted to pack the electrode materials densely, increase the electrical contact between the active material and conductive additives and enhance the adhesion between the active material and the current collector. The pressure involved in the calendering process could reach 80 MPa. Under such a high pressure, lots of nanostructures could collapse, which may limit the practical application of these nanostructures. Fortunately, the mechanical property of the hollow structure could be adjusted to withstand the high pressure by controlling the composition, crystallinity, aggregation of nano subunits, thickness of the shell and the shell number. For example, an amorphous carbon shell is fragile and cracks after only a slight deformation; in contrast, a graphene cage is mechanically strong and flexible and shows much better resilience to the external pressure.27 Besides, metal oxides usually have better mechanical robustness than carbon materials. In situ TEM indentation validates that a TiO2 hollow sphere with a shell thickness of 16 nm could maintain its structural integrity under a high pressure of 160 MPa, while an amorphous carbon hollow sphere collapses under a pressure less than 41 MPa.40 Additionally, it's worth mentioning that the external force could be shared and decentralized by multiple shells of the HoMS, thus endowing the HoMS with much better mechanical robustness than its single-shelled counterpart (Fig. 3b).
As the energy density increases, the concern toward battery safety becomes more severe. Accidents related to fires and explosions of LIBs happen frequently. For example, the Samsung Note 7 fire and Tesla electric car battery fire have drawn worldwide attention.41 As for the Li metal anode, battery safety could be more critical. This is because uncontrolled Li dendrite growth could induce an inner short circuit, which may result in abrupt temperature increase and would eventually induce thermal runaway if no timely action is taken. Burgeoning efforts have been made to ensure battery safety. An external protection approach includes adding a temperature sensor or a pressure valve in batteries, which would sacrifice battery energy density and are not reliable under unfavourable conditions. An internal protection approach relies on safety materials and has been considered as the ultimate choice to build safe batteries.42 A heterogeneous HoMS is promising to resolve the safety issue of lithium metal batteries. As shown in Fig. 3c, on one hand, the inner nucleation shell could guide the Li metal to plate inside the inner void space, while the robust protective shell can inhibit Li dendrite growth and limit Li–electrolyte contact area, thus decreasing the safety risk; on the other hand, the outer thermal-responsive polymer shell could protect the fire-retardant shell from direct exposure to the electrolyte and melting during thermal runaway to release fire-retardant materials, thus avoiding an explosion.
Conclusion and outlook
In this Focus article, we have provided a brief yet insightful explanation that a hollow structured electrode is indispensable to practically realize a high energy density for rechargeable batteries. In addition, we highlight that compared to its single-shelled counterpart, a HoMS-based electrode can provide a higher volumetric capacity and a better mechanical property and enable dynamic smart energy-storage behaviors. Despite that, a HoMS-based electrode is still a proof-of-concept study. To make it commercialized for practical rechargeable batteries, future efforts should be focused on the following aspects.Firstly, to make HoMS-based electrodes for industrial application, the fabrication of HoMSs with controllable structure and composition should be facile, scalable, environmentally friendly and cost-effective. Secondly, to further improve the energy storage application performance and widen the application area, more efforts should be devoted to the fabrication of heterogeneous HoMSs with accurately controlled differential compositions for different shells. Thirdly, the void ratio in HoMSs should be well-designed to ensure enough cavity for volume expansion during lithiation and simultaneously maximize the volumetric energy density. Besides, the adding ratio of the HoMS-based host in the electrode should also be optimized to ensure a high energy density without sacrificing other merits. In addition, the porosity and other parameters of HoMSs should be optimized to ensure good mechanical robustness to survive the calendering process during practical electrode fabrication. Fourthly, loading HoMSs with a three-dimensional conductive host, or coating HoMSs with carbon and conductive polymers is promising to realize an ideal conductive network for electrode application, thus improving the energy storage performance. Last but not the least, to reach a purposeful design of HoMS-based electrodes, a better understanding of the reaction mechanism and structure–performance correlations should be realized through in situ observation assisted with advanced characterization techniques and calculation modeling.
In summary, the future with hollow multishelled structures for rechargeable batteries having a long cycling life and high energy density is bright. With continued efforts devoted to this area, the structure of hollow multishelled structures will be optimized to further improve the performance of high energy density batteries.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21590795, 21820102002 and 51802306) and the Scientific Instrument Developing Project of the Chinese Academy of Sciences (grant no. YZ201623).References
- X. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 RSC.
- J. Qi, X. Y. Lai, J. Y. Wang, H. J. Tang, H. Ren, Y. Yang, Q. Jin, L. J. Zhang, R. B. Yu, G. H. Ma, Z. G. Su, H. J. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- J. Wang, Y. Cui and D. Wang, Adv. Mater., 2018, 1801993 Search PubMed.
- T. Yi, J. Li, Y. Zhang and X. Yang, Chem. Res. Chin. Univ., 2018, 34, 661–664 CrossRef CAS.
- B. Li, J. Huang and X. Wang, Chem. Res. Chin. Univ., 2019, 35, 125–132 CrossRef CAS.
- C. Wang, J. Wang, W. Hu and D. Wang, Chem. Res. Chin. Univ., 2020, 36, 68–73 CrossRef CAS.
- J. Wang, H. Tang, L. Zhang, H. Ren, R. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. Liu, Y. Zhang, Y. Wen, L. Gu, G. Ma, Z. Su, Z. Tang, H. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
- C. Jiao, Z. Wang, X. Zhao, H. Wang, J. Wang, R. Yu and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 996 CrossRef CAS PubMed.
- H. Ren, R. B. Yu, J. Y. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. J. Zhao and D. Wang, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed.
- F. Wang, J. Y. Wang, H. Ren, H. J. Tang, R. B. Yu and D. Wang, Inorg. Chem. Front., 2016, 3, 365–369 RSC.
- X. Zhao, J. Wang, R. Yu and D. Wang, J. Am. Chem. Soc., 2018, 140, 17114–17119 CrossRef CAS PubMed.
- X. X. Zhao, R. B. Yu, H. J. Tang, D. Mao, J. Qi, B. Wang, Y. Zhang, H. J. Zhao, W. P. Hu and D. Wang, Adv. Mater., 2017, 29, 1700550 CrossRef PubMed.
- S. M. Xu, C. M. Hessel, H. Ren, R. B. Yu, Q. Jin, M. Yang, H. J. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 RSC.
- J. Wang, H. Tang, H. Wang, R. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- J. Xie, J. Wang, H. R. Lee, K. Yan, Y. Li, F. Shi, W. Huang, A. Pei, G. Chen, R. Ssubbaraman, J. Christensen and Y. Cui, Sci. Adv., 2018, 4, eaat5168 CrossRef CAS PubMed.
- E. H. M. Salhabi, J. Zhao, J. Wang, M. Yang, B. Wang and D. Wang, Angew. Chem., Int. Ed., 2019, 58, 9078–9082 CrossRef CAS PubMed.
- J. Zhao, M. Yang, N. Yang, J. Wang and D. Wang, Chem. Res. Chin. Univ., 2020, 36, 313–319 CrossRef CAS.
- Q. Liu, Q. Lei, H. Xu and J. Yuan, Resour., Conserv. Recycl., 2018, 128, 78–89 CrossRef.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Nat. Energy, 2018, 3, 267–278 CrossRef CAS.
- J. Wang, L. Liao, Y. Li, J. Zhao, F. Shi, K. Yan, A. Pei, G. Chen, G. Li, Z. Lu and Y. Cui, Nano Lett., 2018, 18, 7060–7065 CrossRef CAS PubMed.
- J. Wang, L. Liao, H. R. Lee, F. Shi, W. Huang, J. Zhao, A. Pei, J. Tang, X. Zheng, W. Chen and Y. Cui, Nano Energy, 2019, 61, 404–410 CrossRef CAS.
- C. K. Chan, H. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- N. Liu, K. Huo, M. T. McDowell, J. Zhao and Y. Cui, Sci. Rep., 2013, 3, 1919 CrossRef PubMed.
- L.-F. Cui, R. Ruffo, C. K. Chan, H. Peng and Y. Cui, Nano Lett., 2009, 9, 491–495 CrossRef CAS PubMed.
- H. Wu, G. Chan, J. W. Choi, I. Ryu, Y. Yao, M. T. McDowell, S. W. Lee, A. Jackson, Y. Yang, L. Hu and Y. Cui, Nat. Nanotechnol., 2012, 7, 310 CrossRef CAS PubMed.
- N. Liu, H. Wu, M. T. McDowell, Y. Yao, C. Wang and Y. Cui, Nano Lett., 2012, 12, 3315–3321 CrossRef CAS PubMed.
- Y. Li, K. Yan, H.-W. Lee, Z. Lu, N. Liu and Y. Cui, Nat. Energy, 2016, 1, 15029 CrossRef CAS.
- Y. Liu, G. Zhou, K. Liu and Y. Cui, Acc. Chem. Res., 2017, 50(12), 2895–2905 CrossRef CAS PubMed.
- G. Zheng, S. W. Lee, Z. Liang, H.-W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu and Y. Cui, Nat. Nanotechnol., 2014, 9, 618–623 CrossRef CAS PubMed.
- K. Yan, Z. Lu, H.-W. Lee, F. Xiong, P.-C. Hsu, Y. Li, J. Zhao, S. Chu and Y. Cui, Nat. Energy, 2016, 1, 16010 CrossRef CAS.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- G. Zhou, D.-W. Wang, F. Li, P.-X. Hou, L. Yin, C. Liu, G. Q. Lu, I. R. Gentle and H.-M. Cheng, Energy Environ. Sci., 2012, 5, 8901–8906 RSC.
- G. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed.
- Z. W. Seh, W. Li, J. J. Cha, G. Zheng, Y. Yang, M. T. McDowell, P.-C. Hsu and Y. Cui, Nat. Commun., 2013, 4, 1331 CrossRef PubMed.
- X. Tao, J. Wang, Z. Ying, Q. Cai, G. Zheng, Y. Gan, H. Huang, Y. Xia, C. Liang, W. Zhang and Y. Cui, Nano Lett., 2014, 14, 5288–5294 CrossRef CAS PubMed.
- J. Y. Wang, J. Wan and D. Wang, Acc. Chem. Res., 2019, 52, 2169–2178 CrossRef CAS PubMed.
- D. Mao, J. Wan, J. Wang and D. Wang, Adv. Mater., 2018, 1802874 Search PubMed.
- J. Wang, J. Wan, N. Yang, Q. Li and D. Wang, Nat. Rev. Chem., 2020, 4, 159–168 CrossRef.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed.
- Y. Jin, S. Li, A. Kushima, X. Zheng, Y. Sun, J. Xie, J. Sun, W. Xue, G. Zhou, J. Wu, F. Shi, R. Zhang, Z. Lu, K. So, Y. Cui and J. Li, Energy Environ. Sci., 2017, 10, 580–592 RSC.
- K. Liu, W. Liu, Y. Qiu, B. Kong, Y. Sun, Z. Chen, D. Zhuo, D. Lin and Y. Cui, Sci. Adv., 2017, 3, e160197 Search PubMed.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Sci. Adv., 2018, 4, eaas982 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |