White luminescent single-crystalline chlorinated graphene quantum dots†
Weitao
Li
a,
Huazhang
Guo
a,
Gao
Li
a,
Zhen
Chi
b,
Hailong
Chen
 b,
Liang
Wang
b,
Liang
Wang
 *ac,
Yijian
Liu
a,
Keng
Chen
a,
Mengying
Le
a,
Yu
Han
a,
Luqiao
Yin
d,
Robert
Vajtai
*ac,
Yijian
Liu
a,
Keng
Chen
a,
Mengying
Le
a,
Yu
Han
a,
Luqiao
Yin
d,
Robert
Vajtai
 ce,
Pulickel M
Ajayan
*c,
Yuxiang
Weng
b and
Minghong
Wu
*f
ce,
Pulickel M
Ajayan
*c,
Yuxiang
Weng
b and
Minghong
Wu
*f
aInstitute of Nanochemistry and Nanobiology, School of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, P. R. China. E-mail: wangl@shu.edu.cn
bBeijing National Laboratory for Condensed Matter Physics, CAS Key Laboratory of Soft Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, P. R. China
cDepartment of Materials Science and NanoEngineering, Rice University, 6100 Main Street, Houston, TX 77005, USA. E-mail: ajayan@rice.edu
dKey Laboratory of Advanced Display and System Applications, Shanghai University, Ministry of Education, Shanghai 200072, P. R. China
eInterdisciplinary Excellence Centre, Department of Applied and Environmental Chemistry, University of Szeged, Rerrich Béla tér 1., Szeged, Hungary
fShanghai Institute of Applied Radiation, Shanghai University, Shanghai 200444, P. R. China. E-mail: mhwu@shu.edu.cn
First published on 16th March 2020
Abstract
A new class of white luminescent materials, white-light-emitting graphene quantum dots (WGQDs), have attracted increasing attention because of their unique features and potential applications. Herein, we designed and synthesized a novel WGQDs VIA a solvothermal molecular fusion strategy. The modulation of chlorine doping amount and reaction temperature gives the WGQDs a single-crystalline structure and bright white fluorescence properties. In particular, the WGQDs also exhibit novel and robust white phosphorescence performance for the first time. An optimum fluorescence quantum yield of WGQDs is 34%, which exceeds the majority of reported WGQDs and other white luminescent materials. The WGQDs display broad-spectrum absorption within almost the entire visible light region, broad full width at half maximum and extend their phosphorescence emission to the entire white long-wavelength region. This unique dual-mode optical characteristic of the WGQDs originates from the synergistic effect of low-defect and high chlorine-doping in WGQDs and enlarges their applications in white light emission devices, cell nuclei imaging, and information encryption. Our finding provides us an opportunity to design and construct more advanced multifunctional white luminescent materials based on metal-free carbon nanomaterials.
New conceptsWhite luminescent materials have been generating much excitement because of their wide-ranging potential applications. However, challenging synthesis, cytotoxicity and performance of current reported white luminescent materials still hinder their potential applications. Owing to their non-toxicity, excellent optical properties, and amenability to surface modification, white-light-emitting graphene quantum dots (WGQDs) are considered to be a next-generation white luminescent material to replace these above-mentioned conventional materials. The inherent challenge in WGQDs is their massive defects are known to give poor white optical properties. In the proof-of-concept, we designed and synthesized a novel WGQDs via a solvothermal molecular fusion route. The modulation of chlorine doping amount and reaction temperature gives the WGQDs a single-crystalline structure, bright white fluorescence and novel white phosphorescence performance for the first time. An optimum fluorescence quantum yield of WGQDs is 34%, which exceeds the majority of reported WGQDs and other white luminescent materials. The WGQDs display broad-spectrum absorption within almost the entire visible light region, broad full width at half maximum and extend their phosphorescence emission to the entire white long-wavelength region. This unique dual-mode optical characteristic of the WGQDs enlarges their applications in white light emission devices, cell nuclei imaging, and information encryption. |
White luminescent materials have been generating much excitement because of their wide-ranging potential applications in displays, white light-emitting diodes (WLEDs), solar cells, photocatalysis, bioimaging, and sensing.1–4 At present, reported white luminescent materials are mainly focused on organic molecules and inorganic phosphors containing rare earth metal elements, such as hybrid semiconductor bulk materials,5 metal–organic frameworks,2 lanthanide-based gels,6 covalent organic frameworks,3 dyes,7 and conjugated polymers.8 Unfortunately, most of the semiconductor materials contain the high-toxicity Cd and Pb elements as the rare earth metals in lanthanide-based gels, causing serious environmental and health issues.5,6 Meanwhile, their synthesis procedures require high energy consumption.6 Besides, the metal–organic frameworks and covalent organic frameworks are eco-friendless due to their low production yields and expensive precursor ligands.2,3 Furthermore, dyes and conjugated polymers still have many shortcomings in luminous efficiency, color purity, and stability, further hindering their potential applications.7,8 Therefore, it is urgent to search for a novel, low-cost, and sustainable white luminescent materials with high performance.
Graphene quantum dots (GQDs) are a popular class in the extensive carbon family.9–13 Owing to their non-toxicity, excellent optical properties, and amenability to surface modification,14,15 white-light-emitting GQDs (WGQDs) are considered to be a next-generation white luminescent material to replace these above-mentioned conventional materials. There are some previous reports about WGQDs, which methods can be roughly divided into two types based on the precursors, top-down method16–20 and bottom-up route.21 The former uses large-sized carbon materials as the precursors, such as graphite,16 carbon black,17 and graphene.19,20 Then the precursors were cut into small-sized WGQDs by a strong acid, which broke their intrinsic sp2-hybridized structure, created massive defects, and resulted in their weak white fluorescence.19 Even if the full width at half maximum (FWHM) of the synthesized WGQDs is 150 nm, their photoluminescence (PL) range falls into the ultraviolet region.16 In contrast, the bottom-up approach makes small molecules to self-assemble construct WGQDs through a series of chemical reactions. Li et al. synthesized WGQDs by hydrothermal reaction using fructose and hydrochloric acid as raw materials.21 Therefore, the quantum yield (QY) of the reported WGQDs is only 6.8%, which limits its extensive potential applications. Despite the fact that the bottom-up way is a benefit for moderating the properties of the WGQDs fitting what we need, there are still shortcomings in current methods for synthesizing high-quality WGQDs that must be addressed.
With this in mind, we designed a simple and general one-pot solvothermal strategy to manufacture a novel WGQDs. By modulating the synthetic conditions, such as reaction temperature and the volume ratio of reaction solvents, the Cl-doping amount and defect degree of WGQDs can be regulated. Excellent dual-mode white fluorescence and phosphorescence characteristics of WGQDs can be achieved simultaneously, making this the first report of WGQDs with phosphorescence properties. A mechanism for the formation of these dual-mode WGQDs is proposed. Based on the dual-mode optical performance, the applications of WGQDs for WLEDs, cell nuclei imaging, and advanced information encryption are successfully demonstrated.
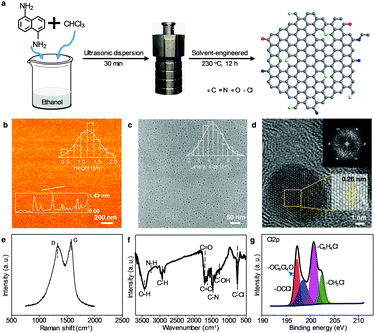
The WGQDs were synthesized by the solvothermal molecular fusion method using 1,5-diaminonaphthalene (DAN) as a precursor and trichloromethane (TCM) as a Cl source (Fig. 1a). By modulating reaction conditions (e.g., temperature, the ratio of ethanol and TCM), the optimum QY of WGQDs was up to 34% (Tables S1 and S2, ESI†) with a certain volume ratio (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 vs. ethanol
4 vs. ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TCM) at temperature of 230 °C for 12 h (Fig. S1–S4, ESI†). This QY of produced WGQDs exceeds the majority of reported WGQDs and other white luminescent materials (Table S3, ESI†).1–3,6,7,17 Besides, the WGQDs exhibits good solvent-dispersibility in many solvents and continues to display white fluorescence performance with a broad FWHM of 138 nm (Fig. S5, S6 and Table S4, ESI†), which allows them to adapt to complex application environments. For comparison, the No-Cl-GQDs and Lt-GQDs were also synthesized for further investigations. The details can be found in the Experimental section.
TCM) at temperature of 230 °C for 12 h (Fig. S1–S4, ESI†). This QY of produced WGQDs exceeds the majority of reported WGQDs and other white luminescent materials (Table S3, ESI†).1–3,6,7,17 Besides, the WGQDs exhibits good solvent-dispersibility in many solvents and continues to display white fluorescence performance with a broad FWHM of 138 nm (Fig. S5, S6 and Table S4, ESI†), which allows them to adapt to complex application environments. For comparison, the No-Cl-GQDs and Lt-GQDs were also synthesized for further investigations. The details can be found in the Experimental section.
The atomic force microscopy (AFM) image of WGQDs (Fig. 1b) reveals three to four layers of graphene with an average thickness of 1.32 nm, counting the thickness of a total of 300 samples. The transmission electron microscopy (TEM) image (Fig. 1c) verifies the uniform-dispersion of WGQDs with an average size of 4.5 nm, which was calculated from about 400 samples. The high-resolution TEM image (Fig. 1d) illustrates the single-crystal structure of WGQDs with an enlarged crystal plane spacing of 0.26 nm due to the surface groups and doping of WGQDs. Similarly, the (002) interlayer spacing of 3.84 Å of WGQDs in the X-ray diffraction patterns (XRD) is also more extensive than that of No-Cl-GQDs (3.63 Å) and Lt-GQDs (3.66 Å) (Fig. S7, ESI†).14,22 Meanwhile, there are two apparent peaks at 1335 and 1580 cm−1 that correspond to the disordered sp3 hybrid carbon (D band) and crystalline sp2 hybrid structure (G band) in the Raman spectrum (Fig. 1e and Fig. S8, ESI†). The WGQDs displays the highest IG/ID ratio of 1.35 in three samples, indicating its low-defect degree. Note that the IG/ID ratio of No-Cl-GQDs (0.86) is lower than that of Lt-GQDs (1.07), demonstrating the Cl-doping plays a dominant role in eliminating their defect. The low-defect structure of WGQDs extends their luminescent feature to the white light region.23
To understand the presence of the ligands, various spectral characterizations were used to test the functional groups of the WGQDs. The sp2 C atoms (100–160 ppm) can be seen in the 13C nuclear magnetic resonance (NMR) spectrum (Fig. S9a, ESI†), further confirming the conclusion of the Raman spectrum. There is obvious aromatic ring hydrogen in the range of 6.5–8.5 ppm in the 1H NMR spectrum (Fig. S9b, ESI†). Besides, active hydrogen signals with two peaks (6.5 and 5.35 ppm) are indicating NH2 and OH groups, respectively, which also appear in the Fourier transform infrared (FT-IR) spectrum (Fig. 1f). Furthermore, the special stretching vibration for C–Cl (746 cm−1) reveals the Cl-doping in WGQDs. Four peaks of C1s (283.88 eV), N1s (397.01 eV), O1s (531.61 eV) and Cl2p (199.57 eV)24 are observed in the X-ray photoelectron spectroscopy (XPS) (Fig. S10a, ESI†), with an atom ratio of 82.63%, 7.03%, 7.84%, and 2.5%, respectively. In comparison to the XPS data of WGQDs, the No-Cl-GQDs don’t contain any Cl element, and the Cl content of Lt-GQDs (1.32%) is lower than that of WGQDs (Fig. S11, S12 and Table S5, ESI†), suggesting the high reaction temperature can increase the Cl-doping content in WGQDs. Moreover, by fitting the C–Cl bands in the high-resolution Cl2p spectrum (Fig. 1g), four peaks of –OCCl (197.2 eV), –OC6Cl4O (199.3 eV), –C6H4Cl (200.5 eV), –CH2Cl (201.9 eV) are obtained,25 evidencing that Cl atoms have four different bonding forms in WGQDs. Combined with the C–Cl (287.2 eV) in the C1s spectrum (Fig. S10b, ESI†), it signifies that the Cl atoms are successfully doped in the crystal of WGQDs and also exist on their surface as the functional groups. Overall, the above test results prove that there are abundant –NH2, –OH and –Cl at the edge of the WGQDs, and the high content of Cl-doping stimulates the formation of WGQDs.
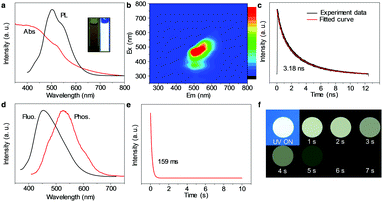
The optical properties of WGQDs are essential to investigate. In Fig. 2a, the UV/Vis absorption of the WGQDs can extend at the edge of 790 nm and covers the entire visible light region. The PL spectrum of WGQDs displays the broad fluorescence emission band with an FWHM of 120 nm, which is consistent with the properties of other white luminescent materials.26 A fluorescence emission peak of 510 nm along with a relatively weak peak at 560 nm can be observed for WGQDs at the excitation of 390 nm. The maximum emissions for WGQDs gradually change from 450 to 600 nm when their excitation wavelengths range from 310 to 550 nm (Fig. 2b), indicating that WGQDs exhibits excitation-dependent properties like the majority of previously reported WGQDs.27–29 The observed lifetime of the WGQDs is 3.18 ns with a single exponential decay characteristic (Fig. 2c). More specifically, the WGQDs exhibits robust stability features, including storage stability, colloidal stability, and photostability in solution (Fig. S13–S15, ESI†). Bandgap energy of the WGQDs (2.07 eV) is calculated using the equation Eoptg = 1240/λedge, where λedge is the onset value of the first excitonic absorption bands in the direction of longer wavelengths.30 In Fig. S16 (ESI†), the ultraviolet photoelectron spectroscopy (UPS) data illustrates the highest occupied molecular orbital (HOMO) level (4.72 eV), and the lowest unoccupied molecular orbital level of 2.65 eV is deduced from the energy gap and HOMO level.
A few published GQD/carbon dots (CDs) displayed phosphorescence properties by means of embedding the GQD/CDs into other matrices (polyvinyl alcohol, urea/biuret or zeolites).31,32 The stability of reversible interactions in these composites is challenging to achieve and thus far, phosphorescence emission of these GQD/CDs-based phosphorescent materials is mainly located in the short-wavelength region (blue- to green-light),33,34 which impeded their broad potential applications. Surprisingly, our pure WGQDs reveals unique white phosphorescence performance without any additives and extends their phosphorescence emission to the entire white long-wavelength region. This is the first report of white phosphorescence performance in the field of GQD/CDs. Compared with the fluorescence properties of WGQDs, the phosphorescence emission peak of WGQDs is an obvious red-shift ranging from 460 to 540 nm, and obtains a relatively broad FWHM of 150 nm (Fig. 2d). In contrast, the phosphorescence spectrum of WGQDs exciting at different wavelengths demonstrates excitation-independent characteristics (Fig. S17, ESI†). The corresponding phosphorescence lifetime (under 390 nm excitation) is 159 ms (Fig. 2e), which is much longer than the fluorescence lifetime (Fig. 2c). The vast distance of their lifetime between phosphorescence and fluorescence is easy to distinguish the optical phenomenon. The long phosphorescence lifetime is a benefit for its broad potential applications. Meanwhile, WGQDs dried on the printing paper can still emit white phosphorescence that is visible to the naked eye for about 7 s after turning off the 365 nm UV light (Fig. 2f).
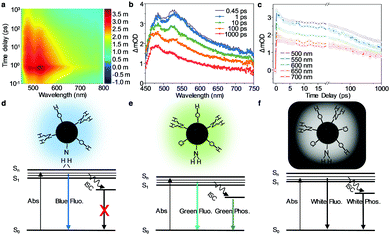
To further investigate the luminescence mechanism of the WGQDs, the advanced optical characterizations of the WGQDs have been measured using femtosecond transient absorption (TA) spectroscopy (Fig. 3 and Fig. S18, ESI†).35,36 In the TA spectra (Fig. 3a and b), the widespread positive (red) features ranging from 450 to 750 nm corresponds to the excited state absorption (ESA) manifesting the transition from the first excited (S1) state to higher electronic states after photoexcitation. A rapid increase in the TA signal after photoexcitation can be observed at different wavelengths, which is followed by a multi-exponential decay feature for all of these data (Fig. 3c). The global analysis of the TA data is used to explore the detailed relaxation channels of the photoinduced carriers, from which four exponential decay components are well fitted (Fig. S18, ESI†). The first three fitted time constants are 3.0 ps, 99 ps, and 3.2 ns, respectively. The fourth component has a lifetime much longer than the current time window for collection, which can be identified as the long-lived phosphorescence emission process. Owing to the previous experience analysis and the fitted decay associated difference spectra and decay dynamics of WGQDs (Fig. S18a and b, ESI†), we attribute the first three components to the corresponding relaxation channels. After excitation, the coulomb-induced hot carriers in the sp2 cluster release their excess energy into the surrounding environment via electron–electron scattering and/or electron-optical-phonon scattering with a time constant of 3.0 ps,37 during which the excitons that can emit white light are formed. Part of the relaxed electron–hole pairs finally recombine via the PL emission, and the remaining carriers will experience the nonradiative transition into the ground state. Two recombination time constants 99 ps and 3.2 ns are observed. The time constant of 3.2 ns is very close to the fitted lifetime from the time-resolved PL spectrum of WGQDs (Fig. 2c), and hence, we assign the corresponding decay component to the PL emission processes. Then, the recombination time constant of 99 ps can be attributed to the nonradiative recombination process. The TA results strongly suggest that the synergistic effect of the sp2 cluster and the surface state contributes to the PL.38,39
In addition to the No-Cl-GQDs and the Lt-GQDs, a third type of controlled sample was synthesized without Cl dopant at relatively low-temperature (180 °C) for the purpose of further verifying the origin of white phosphorescence of WGQDs. The phosphorescence performances of the three samples were measured, shown in the Fig. S19 and S20 (ESI†). The No-Cl-GQDs produced at low or high-temperature show no phosphorescence, revealing that the phenomenon of phosphorescence is not caused by the vacancy of the Cl element. Meanwhile, the Lt-GQDs show weak brown phosphorescence. Therefore, Cl-doping in GQDs plays a crucial role in creating their phosphorescence characteristics by facilitating the intersystem crossing (ISC) for effectively populating triplet excitons (Fig. 3d–f). However, this is not the sole factor responsible for generating the white phosphorescence, which may be related to the defect degree of the GQDs. The vast majority of reported Cl-doping QDs were synthesized by HCl activation of precursors (Table S6, ESI†). Although some GQD/CDs contained high Cl-doping amounts, they exhibited weak fluorescence and never phosphorescence properties due to their high-defect during a violent reaction. Other GQD/CDs produced in mild reactions also didn’t exhibit phosphorescence because their Cl-doping content is low. In summary, the white fluorescence/phosphorescence performance of WGQDs is the result of the combination of low-defect and high Cl-doping, which is consistent with the heavy atomic effect of halogen doping in the reports.40,41 The Cl atoms play an essential role in the cocrystals of the WGQDs, the high nuclear charge of the Cl atoms enhances the spin–orbit coupling of the WGQDs, thereby increasing the probability of conversion between singlet and triplet.
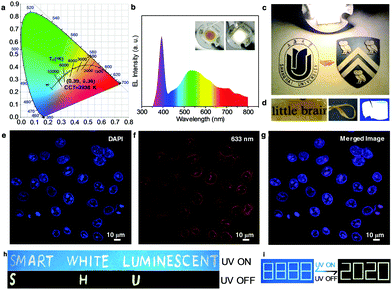
The WGQDs can serve as an ideal phosphor for future high-quality WLEDs due to their unique white light properties. By choosing the different WGQDs synthesized in various TCM volume ratio (Fig. S21 and Table S7, ESI†) and modulating the concentration of the WGQDs (Fig. S22 and Table S8, ESI†), the Commission Internationale del’Eclairage (CIE) color coordinates of WLEDs and correlated parameters can be converted. After the optimization procedure, the CIE color coordinates of the WLED lamp with correlated color temperature (CCT) of 3938 K is (0.39, 0.38) (Fig. 4a), which is marked in CIE 1931 color space and the color point is laid on the black body Planckian locus, approaching the warm white-light region.42 Meanwhile, the WLEDs using pure WGQDs as the phosphor emitted bright warm white light (Insert in Fig. 4b) with color rendering index (CRI) of 70.6, and achieved a high luminous efficiency of 14.92 lm W−1 at 30 mA current, which is comparable to some state of the art WLEDs based on semiconductor QDs and rare-earth phosphors (Table S3, ESI†).1,5 The corresponding emission spectrum of warm WLED consists of two apparent peaks centered at 390 and 530 nm, which can be assigned to the emission of UV LED chip and WGQD phosphor (Fig. 4b). Meanwhile, three weak peaks located at 620, 710, and 760 nm are observed and originated from WGQDs. The warm WLED lamp as a light source can illuminate the entire picture brightly, revealing the excellent lighting effect of the working WLED (Fig. 4c). Additionally, Fig. 4d depicts a photograph of the WGQD/ultraviolet rays film, highlighting their flexible fluorescence features, which demonstrates highly flexible film with perfectly transparent without aggregation of WGQDs under daylight and illuminates white light under UV light excitation, reflecting their robust potential applications in flexible displays and other flexible photoelectronic devices.
Besides the superior performance in WLEDs, it is believed that the WGQDs displays robust ability in bio-imaging due to their broad PL emission. The HeLa cells uptake the WGQDs exhibit bright fluorescence in their cell nucleus under different excitation wavelengths (Fig. 4e–g and Fig. S23, ESI†), which is determined by the DAPI co-staining (Fig. 4e). Meaningfully, in comparison with the cell imaging application of Lt-GQDs,22 the synthesized WGQDs reveals excellent cell imaging performance comparable to commercial dye (DAPI) at 633 nm (Fig. 4f and Fig. S24, ESI†), which can observe the cell imaging using GQDs or CDs as fluorescent probes at this long excitation wavelength for the first time, avoiding short-lived background fluorescence effectively. Furthermore, the biocompatibility of WGQDs still exhibits low-toxicity (Fig. S25, ESI†), denoting the enormous potential applications of the WGQDs in the field of bioimaging and biomedicine in the future.
Because of the unique and excellent white phosphorescence properties of the WGQDs, this revolutionary material can be used in information encryption field. The printing ink mixing the white fluorescent GQDs (no phosphorescence)19 with WGQDs solution was used to print a picture. As shown in Fig. 4h, “smart white luminescent” is visible under 365 nm UV illumination. Once the lamp is turned off, “SHU” (Shorthand of Shanghai University) of the white color is revealed as a result of the intrinsic emission differences between PL and phosphorescence. As shown in Fig. 4i, the encryption information printed on paper is not be identified either under daylight (it was nearly colorless nature of the inks) or under a UV lamp (it showed the wrong information with a white pattern of “8888”). When switching off the excitation, the correct information (“2020”) in white color can be seen clearly. In view of the inherent merits of this white phosphorescent material, the WGQDs may hold great promise for advanced information encryption application.
Conclusions
In summary, WGQDs with dual-mode white fluorescence/phosphorescence properties were first synthesized by a solvothermal method through the regulation of Cl-doping amount and reaction temperature in the synthetic process. The WGQDs with a high fluorescence QY of 34% have broad-spectrum absorption (790 nm), wide emission response (FWHM = 120 nm) but also exhibit excellent white phosphorescence, which is reported the white phosphorescence properties of GQDs for the first time. This unique dual-mode optical characteristic of WGQDs originates from the synergistic effect of low-defect and high Cl-doping in WGQDs. Their dual-mode optical properties of WGQDs have been demonstrated to have great potential in WLEDs and nuclear imaging, and what's more, in advanced information encryption. Among these applications, the WGQDs as the fluorescent probe at the long excitation wavelength (633 nm) for cell imaging, and white phosphorescent ink for information encryption are reported for the first time. This work is the first report of dual-mode white fluorescent/phosphorescent materials based on GQDs that can be modulated in their synthesis procedure, serving as a convenient way to design and manufacture new classes of white luminescent materials and may expand their applications in solar cells, photocatalysts, and sensing.Author contributions
L. Wang, M. Wu and P. M. Ajayan conceived the idea, wrote the paper, and provided input with data analysis and discussion. W. Li and G. Li realized material synthesis. H. Guo and Y. Liu performed NMR measurement and analysis. Z. Chi, H. Chen, Y. Weng carried out the femtosecond transient absorption spectroscopy measurement. R. Vajtai and H. Chen helped to analyse the data modify the paper. All authors contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was funded by National Natural Science Foundation of China (No. 21671129, 21901154, 21571124, 21671131, 51605272), the Shanghai Sailing Program (No. 16YF1404400), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT17R71). This work was supported by the Open Fund of Key Laboratory of Advanced Display and System Applications of Ministry of Education, Shanghai University. We thank the Laboratory for Microstructures of Shanghai University.References
- A. Sebastian, M. K. Mahato and E. Prasad, Soft Matter, 2019, 15, 3407–3417 RSC.
- Y. Liu, M. Pan, Q.-Y. Yang, L. Fu, K. Li, S.-C. Wei and C.-Y. Su, Chem. Mater., 2012, 24, 1954–1960 CrossRef CAS.
- S. Haldar, D. Chakraborty, B. Roy, G. Banappanavar, K. Rinku, D. Mullangi, P. Hazra, D. Kabra and R. Vaidhyanathan, J. Am. Chem. Soc., 2018, 140, 13367–13374 CrossRef CAS PubMed.
- R. Gautier, F. Massuyeau, G. Galnon and M. Paris, Adv. Mater., 2019, 31, 1807383 CrossRef PubMed.
- M. Roushan, X. Zhang and J. Li, Angew. Chem., Int. Ed., 2012, 51, 436–439 CrossRef CAS PubMed.
- L. Qiu, C. Yu, X. Wang, Y. Xie, A. M. Kirillov, W. Huang, J. Li, P. Gao, T. Wu, X. Gu, Q. Nie and D. Wu, Inorg. Chem., 2019, 58, 4524–4533 CrossRef CAS PubMed.
- Z. He, W. Zhao, J. W. Y. Lam, Q. Peng, H. Ma, G. Liang, Z. Shuai and B. Z. Tang, Nat. Commun., 2017, 8, 416 CrossRef PubMed.
- E. J. W. List, G. Leising, N. Schulte, D. A. Schluer, U. Scherf and W. Graupner, Jpn. J. Appl. Phys., 2000, 39, L760–L762 CrossRef CAS.
- M. Zhao, D. Kim, V. L. Nguyen, J. Jiang, L. Sun, Y. H. Lee and H. Yang, Nano Lett., 2019, 19, 61–68 CrossRef CAS PubMed.
- Q. Zhang, S. N. Deng, J. L. Liu, X. X. Zhong, J. He, X. F. Chen, B. W. Feng, Y. F. Chen and K. Ostrikov, Adv. Funct. Mater., 2019, 29, 1805860 Search PubMed.
- Y. Yan, J. Gong, J. Chen, Z. Zeng, W. Huang, K. Pu, J. Liu and P. Chen, Adv. Mater., 2019, 31, 1808283 CrossRef PubMed.
- Y. C. Wang, U. Kadiyala, Z. B. Qu, P. Elvati, C. Altheim, N. A. Kotov, A. Violi and J. S. VanEpps, ACS Nano, 2019, 13, 4278–4289 CrossRef CAS PubMed.
- H. Y. Shan, Y. Yu, R. Zhang, R. T. Cheng, D. Zhang, Y. Luo, X. L. Wang, B. W. Li, S. Zu, F. Lin, Z. Liu, K. Chang and Z. Y. Fang, Mater. Today, 2019, 24, 10–16 CrossRef CAS.
- L. Wang, Y. Wang, T. Xu, H. Liao, C. Yao, Y. Liu, Z. Li, Z. Chen, D. Pan, L. Sun and M. Wu, Nat. Commun., 2014, 5, 5357 CrossRef CAS PubMed.
- Y. Yan, J. Chen, N. Li, J. Tian, K. Li, J. Jiang, J. Liu, Q. Tian and P. Chen, ACS Nano, 2018, 12, 3523–3532 CrossRef CAS PubMed.
- R. Sekiya, Y. Uemura, H. Murakami and T. Haino, Angew. Chem., Int. Ed., 2014, 53, 5619–5623 CrossRef CAS PubMed.
- Z. M. Luo, G. Q. Qi, K. Y. Chen, M. Zou, L. H. Yuwen, X. W. Zhang, W. Huang and L. H. Wang, Adv. Funct. Mater., 2016, 26, 2739–2744 CrossRef CAS.
- X. F. Wang, G. G. Wang, J. B. Li, Z. Liu, Y. X. Chen, L. F. Liu and J. C. Han, Chem. Eng. J., 2019, 361, 773–782 CrossRef CAS.
- T. Ghosh and E. Prasad, J. Phys. Chem. C, 2015, 119, 2733–2742 CrossRef CAS.
- P. Dong, B. P. Jiang, W. Q. Liang, Y. Huang, Z. J. Shi and X. C. Shen, Inorg. Chem. Front., 2017, 4, 712–718 RSC.
- X. M. Li, S. P. Lau, L. B. Tang, R. B. Ji and P. Z. Yang, J. Mater. Chem. C, 2013, 1, 7308–7313 RSC.
- L. Wang, B. Wu, W. T. Li, S. L. Wang, Z. Li, M. Li, D. Y. Pan and M. H. Wu, Adv. Biosyst., 2018, 2, 1700191 CrossRef.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed.
- D. Zhou, P. T. Jing, Y. Wang, Y. C. Zhai, D. Li, Y. Xiong, A. V. Baranov, S. N. Qu and A. L. Rogach, Nanoscale Horiz., 2019, 4, 388–395 RSC.
- Y. M. Zhang, J. H. Zhao, H. L. Sun, Z. Q. Zhu, J. Zhang and Q. J. Liu, Sens. Actuators, B, 2018, 266, 364–374 CrossRef CAS.
- L. H. Mao, W. Q. Tang, Z. Y. Deng, S. S. Liu, C. F. Wang and S. Chen, Ind. Eng. Chem. Res., 2014, 53, 6417–6425 CrossRef CAS.
- L. Wang, W. T. Li, B. Wu, Z. Li, D. Y. Pan and M. H. Wu, Chem. Eng. J., 2017, 309, 374–380 CrossRef CAS.
- L. Wang, W. T. Li, B. Wu, Z. Li, S. L. Wang, Y. Liu, D. Y. Pan and M. H. Wu, Chem. Eng. J., 2016, 300, 75–82 CrossRef CAS.
- W. T. Li, M. Li, Y. J. Liu, D. Y. Pan, Z. Li, L. Wang and M. H. Wu, ACS Appl. Nano Mater., 2018, 1, 1623–1630 CrossRef CAS.
- F. Yuan, Z. Wang, X. Li, Y. Li, Z. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef PubMed.
- S. Mitra, S. Chandra, P. Patra, P. Pramanik and A. Goswami, J. Mater. Chem., 2011, 21, 17638–17641 RSC.
- Y. Deng, D. Zhao, X. Chen, F. Wang, H. Song and D. Shen, Chem. Commun., 2013, 49, 5751–5753 RSC.
- Y. Su, S. Z. F. Phua, Y. Li, X. Zhou, D. Jana, G. Liu, W. Q. Lim, W. K. Ong, C. Yang and Y. Zhao, Sci. Adv., 2018, 4, eaas9732 CrossRef PubMed.
- Y. H. Chen, J. L. He, C. F. Hu, H. R. Zhang, B. F. Lei and Y. L. Liu, J. Mater. Chem. C, 2017, 5, 6243–6250 RSC.
- S. Lu, G. Xiao, L. Sui, T. Feng, X. Yong, S. Zhu, B. Li, Z. Liu, B. Zou, M. Jin, J. S. Tse, H. Yan and B. Yang, Angew. Chem., Int. Ed., 2017, 56, 6187–6191 CrossRef CAS PubMed.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- B. Gao, G. Hartland, T. Fang, M. Kelly, D. Jena, H. G. Xing and L. Huang, Nano Lett., 2011, 11, 3184–3189 CrossRef CAS PubMed.
- Q. Li, T. Y. Luo, M. Zhou, H. Abroshan, J. Huang, H. J. Kim, N. L. Rosi, Z. Shao and R. Jin, ACS Nano, 2016, 10, 8385–8393 CrossRef CAS PubMed.
- L. Sui, W. Jin, S. Li, D. Liu, Y. Jiang, A. Chen, H. Liu, Y. Shi, D. Ding and M. Jin, Phys. Chem. Chem. Phys., 2016, 18, 3838–3845 RSC.
- S. d’Agostino, F. Grepioni, D. Braga and B. Ventura, Cryst. Growth Des., 2015, 15, 2039–2045 CrossRef.
- H. Wang, R. X. Hu, X. Pang, H. Y. Gao and W. J. Jin, CrystEngComm, 2014, 16, 7942–7948 RSC.
- Z. Tian, P. Tian, X. Zhou, G. Zhou, S. Mei, W. Zhang, X. Zhang, D. Li, D. Zhou, R. Guo, S. Qu and A. L. Rogach, Nanoscale, 2019, 11, 3489–3494 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00053a |
| This journal is © The Royal Society of Chemistry 2020 |