Open Access Article
Open Access ArticleAdaptive molecular quaternary clips made with pyrene functionalized polyhedral oligomeric silsesquioxane†
Tsung-Kai
Yang‡
a,
Jou-Tsen
Ou‡
 a,
Heng-Yi
Lin
ab,
Wei-Cheng
Peng
a,
Meng-Hsuan
Jao
a,
Jia
Chen
c,
Bin
Sun
a,
Heng-Yi
Lin
ab,
Wei-Cheng
Peng
a,
Meng-Hsuan
Jao
a,
Jia
Chen
c,
Bin
Sun
 c,
Yu
Zhu
b and
Chien-Lung
Wang
c,
Yu
Zhu
b and
Chien-Lung
Wang
 *a
*a
aDepartment of Applied Chemistry, National Chiao Tung University, 1001 University Rd., Hsinchu, Taiwan 30010. E-mail: kclwang@nctu.edu.tw
bDepartment of Polymer Science, Department of Polymer Engineering, University of Akron, Akron, Ohio 44325, USA
cState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, China
First published on 11th November 2020
Abstract
Evolving synthetic molecules toward complex structures is a major goal in supramolecular chemistry. Increasing the number of clips in a unimolecular multi-clip (UMC), although vital to elevate the complexity of supramolecular architectures, often prevents the UMC from forming host–guest complexes in the bulk phase. To overcome this difficulty, adaptive chemistry was applied to develop a novel adaptive unimolecular quaternary clip (Q-clip). The Q-clip is intrinsically amorphous, but self-organizes with exclusively 4 eq. of allosteric activators (NDI) to form the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complexes and a supramolecular lamellar structure in the bulk. The adaptive assembly is fast and allows us to locate the adaptive assembly area of Q-clip
NDI4 complexes and a supramolecular lamellar structure in the bulk. The adaptive assembly is fast and allows us to locate the adaptive assembly area of Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complexes in the amorphous Q-clip film. Our results provide new insights into the design of adaptive UMCs for the evolution toward complex structures and supramolecular functional materials.
NDI4 complexes in the amorphous Q-clip film. Our results provide new insights into the design of adaptive UMCs for the evolution toward complex structures and supramolecular functional materials.
Host–guest chemistry has been a major topic in supramolecular chemistry since the concept was established in the 1980s.1 Through the precise control over geometrical complementary and the non-covalent interactions between hosts and guest molecules, host–guest chemistry has been applied for mimicking biological processes, developing nanotechnology, and materials science.2–4 Molecular clips are one major type of host molecules.5 Made with pairs of capture units connected to a linker unit, molecular clips pinch guests by their capture units to form host/guest complexes.5 The facile synthesis and high solubility of molecular clips also make them versatile building blocks for supramolecular architectures.6
Increasing the number of the binding sites of a host molecule is essential to evolve synthetic molecules toward complex structures.7 Although molecular di-clips have demonstrated the ability to form more complex supramolecular structures than mono-clips,8–11 further increases in the number of clips in unimolecular multi-clips (UMC) remain elusive. Particularly in the bulk phase, although Nolte et al. pointed out that a bulk assembly of molecular clips is the basis for developing molecular materials with tunable properties, only very few mono-clips,11,12 and no di-clips or UMCs demonstrated host–guest assembly in the bulk phase.
Bulk self-organization of UMCs is difficult due to the high structural complexity of UMCs. To host N guest molecules, a UMC requires 2 N capture units.5 For example, a unimolecular quaternary clip (Q-clip) requires 8 capture units to host 4 guest molecules. Although star-molecules or dendrimers provide sufficient capture units for a UMC, our previous study showed that high conformational freedom complicates the self-organization pathways.13–15 To form ordered bulk structures, these molecules have to sort their high number of conformational isomers into suitable ones to self-organize. Therefore, a large number of capture units could make self-organization too complicated to occur and result in only an amorphous phase.
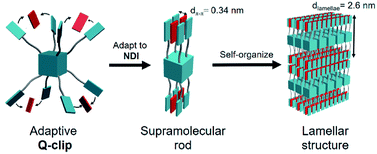
In this study, we developed an amorphous Q-clip that self-organizes in the bulk phase via adapting to guest molecules. As shown in Fig. 1, the Q-clip is an eight-arm-star giant molecule with 8 pyrene capture units connected to a polyhedral oligomeric silsesquioxane (POSS) core.16 The diameter of POSS (ca. 0.7 nm) is about 2 times the d-spacing of π–π stacking (dπ–π ∼ 0.35 nm), which may create suitable space to accommodate a conjugated guest molecule. Although synthetic methods for octa-substituted POSSs have been established in the literature,17–19 the host–guest chemistry of octa-substituted POSS has not yet been developed. To reduce the conformational complexity of the Q-clip and enable the host–guest assembly, N,N′-dihexyl-1,4,6,8-naphthalene diimide (NDI) was used as a positive allosteric effector to unify the conformers of the Q-clip.20 The strong charge transfer (CT) interaction21 between the pyrene capture units of the Q-clip and the NDI guests is utilized to drive the adaptive self-organization of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixtures.22,23 Our results show that adequate geometrical arrangement of the capture units, suitable host/guest interaction, and adaptiveness of the UMC are essential for the design of UMCs that can self-organize in the bulk phase.
NDI mixtures.22,23 Our results show that adequate geometrical arrangement of the capture units, suitable host/guest interaction, and adaptiveness of the UMC are essential for the design of UMCs that can self-organize in the bulk phase.
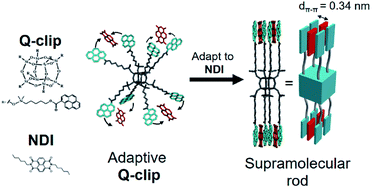 | ||
| Fig. 1 The schematic representation for the conformational change of the Q-clip by the host–guest interaction. | ||
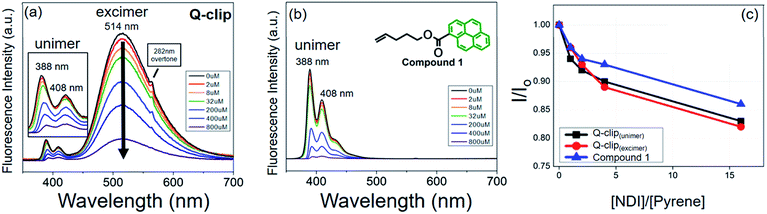
The Q-clip and NDI were synthesized according to Scheme S1.† The products have been fully characterized by 1H NMR, 13C NMR, and mass spectrometry, as shown in Fig. S1–S9.† The geometrical effect of the POSS core can be seen in fluorescence (FL) titration experiments. In Fig. 2a, the Q-clip gives strong excimer FL but weak unimer FL, whereas the pyrene capture unit shows only the unimer FL (Fig. 2b). The aggregated pyrene units of the Q-clip resulted in the stronger CT interaction with the NDI guests,21 as the added NDI quenches the FL of the Q-clip more effectively than compound 1 (Fig. 2c). The unique features of the Q-clip as an adaptive molecular clip and NDI as an allosteric effector in bulk self-organization were further characterized by X-ray diffraction (XRD) and differential scanning calorimetry (DSC). Unlike most of the host molecules that are crystalline,24,25 the Q-clip is an amorphous liquid as evident from the amorphous halos in the XRD pattern and the lack of endothermic transition in the DSC thermograph (green lines in Fig. 3). On the other hand, the NDI guest is a crystalline solid that gives multiple diffraction peaks and endothermic transitions in XRD and DSC measurements (red lines in Fig. 3). As for the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixtures, the presence of NDI triggers the host–guest assembly. The 1
NDI mixtures, the presence of NDI triggers the host–guest assembly. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 Q-clip
4 Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixture gave three diffraction peaks at 2θ = 3.4°, 6.8°, and 10.4°, indicating that each Q-clip pinches 4 equivalents (eq.) of the NDI guest to self-organize into a lamellar structure with a lamellar spacing (dlamellae) of 26 Å. Moreover, the diffraction peak at 2θ = 26° indicates that the pyrene capture units and the NDI guests form orderly packed CT complexes with a π–π stacking distance (dπ–π) of 3.4 Å.21
NDI mixture gave three diffraction peaks at 2θ = 3.4°, 6.8°, and 10.4°, indicating that each Q-clip pinches 4 equivalents (eq.) of the NDI guest to self-organize into a lamellar structure with a lamellar spacing (dlamellae) of 26 Å. Moreover, the diffraction peak at 2θ = 26° indicates that the pyrene capture units and the NDI guests form orderly packed CT complexes with a π–π stacking distance (dπ–π) of 3.4 Å.21
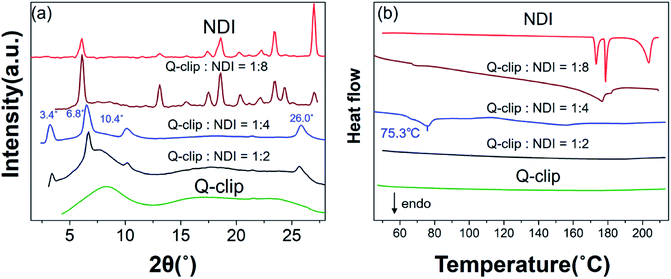 | ||
Fig. 3 (a) The XRD patterns of the Q-clip, NDI, and Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixtures. (b) The DSC thermograms of the Q-clip, NDI, and Q-clip NDI mixtures. (b) The DSC thermograms of the Q-clip, NDI, and Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixtures in the heating course. NDI mixtures in the heating course. | ||
The supramolecular structure of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complexes is illustrated in Fig. 4. The characterization data indicate that the amorphous Q-clip has to adapt to 4 eq. of NDI guests and use the pyrene
NDI4 complexes is illustrated in Fig. 4. The characterization data indicate that the amorphous Q-clip has to adapt to 4 eq. of NDI guests and use the pyrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI CT interaction as the driving force to self-assemble in the bulk state. Goodby et al. showed that octa-substituted POSS derivatives modified with liquid crystalline (LC) mesogens can self-organize into LC phases.26,27 In the smectic LC phases, the octa-substituted POSS derivatives take a rod-conformation and align the long-axes of the rod conformers to form lamellar structures with long-range molecular orientation order. The amorphous Q-clip is different from these LC POSS derivatives because it must adapt to the NDI guests to self-organize into the lamellar structure. The conformational isomerization of the Q-clip involves 4 NDI guest molecules pinched by the eight pyrene capture units. This unique adaptive host–guest assembly thus renders the Q-clip the first example of the adaptive UMC that self-organizes in the bulk phase. Noteworthily, mixing 4 eq. of pyrene with the Q-clip did not activate the adaptive assembly since the amorphous halo of the Q-clip can still be found in the XRD pattern of the Q-clip
NDI CT interaction as the driving force to self-assemble in the bulk state. Goodby et al. showed that octa-substituted POSS derivatives modified with liquid crystalline (LC) mesogens can self-organize into LC phases.26,27 In the smectic LC phases, the octa-substituted POSS derivatives take a rod-conformation and align the long-axes of the rod conformers to form lamellar structures with long-range molecular orientation order. The amorphous Q-clip is different from these LC POSS derivatives because it must adapt to the NDI guests to self-organize into the lamellar structure. The conformational isomerization of the Q-clip involves 4 NDI guest molecules pinched by the eight pyrene capture units. This unique adaptive host–guest assembly thus renders the Q-clip the first example of the adaptive UMC that self-organizes in the bulk phase. Noteworthily, mixing 4 eq. of pyrene with the Q-clip did not activate the adaptive assembly since the amorphous halo of the Q-clip can still be found in the XRD pattern of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrene mixture (Fig. S12†). Thus, the adaptive assembly of the Q-clip requires the NDI
pyrene mixture (Fig. S12†). Thus, the adaptive assembly of the Q-clip requires the NDI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrene CT interaction rather than the pyrene
pyrene CT interaction rather than the pyrene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrene π–π interaction to activate.
pyrene π–π interaction to activate.
 | ||
| Fig. 4 The illustration of the adaptive host–guest assembly of the Q-clip and 4 eq. of NDI guests in the bulk phase. | ||
The second unique feature of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI complex is regarding the allosteric effect of the NDI guest. In Fig. 3a, it was found that 4 eq. of NDI consumed almost all the Q-clip in the host–guest assembly, while 2 eq. of NDI resulted in a very similar diffraction pattern with a residual amorphous Q-clip. The result suggests that even 2 eq. of NDI promoted the formation of the Q-clip
NDI complex is regarding the allosteric effect of the NDI guest. In Fig. 3a, it was found that 4 eq. of NDI consumed almost all the Q-clip in the host–guest assembly, while 2 eq. of NDI resulted in a very similar diffraction pattern with a residual amorphous Q-clip. The result suggests that even 2 eq. of NDI promoted the formation of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex rather than other supramolecular entities. Thus, the Q-clip is an exclusive unimolecular quaternary clip, which either adapts to 4 NDI guests or not at all, and NDI is an allosteric activator that prevents all self-organization pathways other than the formation of the Q-clip
NDI4 complex rather than other supramolecular entities. Thus, the Q-clip is an exclusive unimolecular quaternary clip, which either adapts to 4 NDI guests or not at all, and NDI is an allosteric activator that prevents all self-organization pathways other than the formation of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex.
NDI4 complex.
Finally, the excess eq. of NDI in the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixture prevents the formation of the Q-clip
NDI mixture prevents the formation of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex. In Fig. 3a, the XRD pattern of the 1
NDI4 complex. In Fig. 3a, the XRD pattern of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 Q-clip
8 Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixture (brown line) contains the diffraction peaks of NDI and the amorphous halos of the Q-clip. No characteristic peak of the Q-clip
NDI mixture (brown line) contains the diffraction peaks of NDI and the amorphous halos of the Q-clip. No characteristic peak of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex was detected. In addition, in Fig. 3b, the endothermic phase transition of the supramolecular entity at 75.3 °C is also gone. The results indicate that the excess amount of NDI promotes the crystallization of the NDI guest and suppresses the host–guest assembly completely. The distinct morphologies of the 1
NDI4 complex was detected. In addition, in Fig. 3b, the endothermic phase transition of the supramolecular entity at 75.3 °C is also gone. The results indicate that the excess amount of NDI promotes the crystallization of the NDI guest and suppresses the host–guest assembly completely. The distinct morphologies of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mixtures thus indicate that the crystallization of NDI and the host–guest assembly of the Q-clip
8 mixtures thus indicate that the crystallization of NDI and the host–guest assembly of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI mixture are two competitive routes that lead to different ordered phases whose stability depends on the Q-clip
NDI mixture are two competitive routes that lead to different ordered phases whose stability depends on the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI ratio.
NDI ratio.
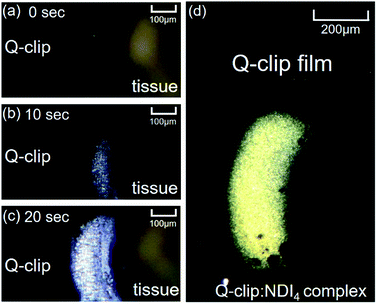
Additional features regarding the adaptive assembly were observed in in situ POM micrographs shown in Fig. 5. Via supplying the allosteric activators (NDI solution) from the tip of the tissue, the amorphous Q-clip quickly assembled with NDI and formed the birefringent area of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex within 20 seconds. The fast adaptive assembly allows us to locate the assembly area on the amorphous Q-clip film. When the allosteric activators were supplied from the top of the Q-clip film via a thin glass capillary, the selective-area growth of the Q-clip
NDI4 complex within 20 seconds. The fast adaptive assembly allows us to locate the assembly area on the amorphous Q-clip film. When the allosteric activators were supplied from the top of the Q-clip film via a thin glass capillary, the selective-area growth of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complexes resulted in the birefringent area in Fig. 5d, and left the untreated area dark. Since the assembly area can be determined by where the allosteric activator is applied, the Q-clip may have the potential to be used as a functional material in micro-/nano-fabrications, where the patterns of the Q-clip
NDI4 complexes resulted in the birefringent area in Fig. 5d, and left the untreated area dark. Since the assembly area can be determined by where the allosteric activator is applied, the Q-clip may have the potential to be used as a functional material in micro-/nano-fabrications, where the patterns of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complex can be created by micro-/nano-stamps.28,29
NDI4 complex can be created by micro-/nano-stamps.28,29
In summary, a Q-clip that carries out the adaptive host–guest assembly in the bulk phase was developed. The Q-clip is an eight-arm-star giant molecule consisting of a POSS core and 8 pyrene capture units. It is intrinsically amorphous due to its high conformational freedom but can pinch exclusively 4 eq. of the allosteric activator, NDI, to form the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 host–guest complex in the bulk phase. In the host–guest complex, the pyrene capture units of the Q-clip and the NDI guests use CT interactions to form continuous π-stacking, which reduces the conformational freedom of the host and assists the amorphous Q-clip to pack into a supramolecular lamellar structure. Increasing the number of binding sites of a host molecule is an important step to evolve host molecules toward complex structures.7 Our study breaks the limits on the number of clips in a UMC and overcomes the obstacles posed by the increased molecular complexity. Moreover, the rapid adaptive assembly of the Q-clip and NDI in the bulk phase allows us to locate the area of the Q-clip
NDI4 host–guest complex in the bulk phase. In the host–guest complex, the pyrene capture units of the Q-clip and the NDI guests use CT interactions to form continuous π-stacking, which reduces the conformational freedom of the host and assists the amorphous Q-clip to pack into a supramolecular lamellar structure. Increasing the number of binding sites of a host molecule is an important step to evolve host molecules toward complex structures.7 Our study breaks the limits on the number of clips in a UMC and overcomes the obstacles posed by the increased molecular complexity. Moreover, the rapid adaptive assembly of the Q-clip and NDI in the bulk phase allows us to locate the area of the Q-clip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NDI4 complexes on the Q-clip film and provides the potential for the supramolecular complex to be used as a functional material in micro-/nano-fabrications. These results provide new insights into the design of adaptive UMCs for the development toward complex matters and supramolecular functional materials.
NDI4 complexes on the Q-clip film and provides the potential for the supramolecular complex to be used as a functional material in micro-/nano-fabrications. These results provide new insights into the design of adaptive UMCs for the development toward complex matters and supramolecular functional materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 106-2221-E-009-130-MY3 and MOST 107-2221-E-009-041-MY3). The authors thank the National Synchrotron Radiation Center for supporting us to carry out structural characterization.Notes and references
- J.-M. Lehn, Angew. Chem., Int. Ed., 1988, 27, 89–112 CrossRef.
- J.-M. Lehn, Chem. Soc. Rev., 2017, 46, 2378–2379 RSC.
- K. Tashiro and T. Aida, Chem. Soc. Rev., 2007, 36, 189–197 RSC.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 CrossRef CAS.
- M. Hardouin Lerouge, P. Hudhomme and M. Sallé, Chem. Soc. Rev., 2011, 40, 30–43 RSC.
- F.-G. Klärner and B. Kahlert, Acc. Chem. Res., 2003, 36, 919–932 CrossRef.
- J.-M. Lehn, Science, 1985, 227, 849–856 CrossRef CAS.
- Y. Tanaka, K. M.-C. Wong and V. W.-W. Yam, Chem.–Eur. J., 2012, 19, 390–399 CrossRef.
- Y.-K. Tian, Y.-G. Shi, Z.-S. Yang and F. Wang, Angew. Chem., Int. Ed., 2014, 53, 6090–6094 CrossRef CAS.
- Y. Tanaka, K. M.-C. Wong and V. W.-W. Yam, Angew. Chem., Int. Ed., 2013, 52, 14117–14120 CrossRef CAS.
- J. L. M. van Nunen and R. J. M. Nolte, J. Chem. Soc., Perkin Trans. 2, 1997, 1473–1478 RSC.
- S. J. Holder, J. A. A. W. Elemans, A. E. Rowan, R. J. M. Nolte and J. Barberá, Chem. Commun., 2000, 355–356 RSC.
- S.-L. Wu, C.-Y. Hong, K.-Y. Wu, S.-T. Lan, C.-T. Hsieh, H.-L. Chen and C.-L. Wang, Chem.–Asian J., 2016, 11, 2011–2015 CrossRef CAS.
- C.-L. Wang, S. P. Prakoso and S.-L. Wu, J. Chin. Chem. Soc., 2018, 65, 368–374 CrossRef CAS.
- J.-T. Weng, T.-F. Yeh, A. Z. Samuel, Y.-F. Huang, J.-H. Sie, K.-Y. Wu, C.-H. Peng, H.-O. Hamaguchi and C.-L. Wang, Nanoscale, 2018, 10, 3509–3517 RSC.
- W.-B. Zhang, X. Yu, C.-L. Wang, H.-J. Sun, I.-F. Hsieh, Y. Li, X.-H. Dong, K. Yue, R. Van Horn and S. Z. D. Cheng, Macromolecules, 2014, 47, 1221–1239 CrossRef CAS.
- M. Y. Lo, C. Zhen, M. Lauters, G. E. Jabbour and A. Sellinger, J. Am. Chem. Soc., 2007, 129, 5808–5809 CrossRef CAS.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS.
- S. Schäfer and G. Kickelbick, Dalton Trans., 2017, 46, 221–226 RSC.
- L. Kovbasyuk and R. Krämer, Chem. Rev., 2004, 104, 3161–3188 CrossRef CAS.
- M. D. Gujrati, N. S. S. Kumar, A. S. Brown, B. Captain and J. N. Wilson, Langmuir, 2011, 27, 6554–6558 CrossRef CAS.
- J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC.
- J.-M. Lehn, Angew. Chem., Int. Ed., 2015, 54, 3276–3289 CrossRef CAS.
- P. van der Asdonk and P. H. J. Kouwer, Chem. Soc. Rev., 2017, 46, 5935–5949 RSC.
- M. T. Sims, L. C. Abbott, S. J. Cowling, J. W. Goodby and J. N. Moore, Chem.–Eur. J., 2015, 21, 10123–10130 CrossRef CAS.
- G. H. Mehl and J. W. Goodby, Angew. Chem., Int. Ed., 1996, 35, 2641–2643 CrossRef CAS.
- J. W. Goodby, R. J. Mandle, E. J. Davis, T. Zhong and S. J. Cowling, Liq. Cryst., 2015, 42, 593–622 CrossRef CAS.
- X. M. Zhao, Y. N. Xia and G. M. Whitesides, J. Mater. Chem., 1997, 7, 1069–1074 RSC.
- A. A. Yu, T. Savas, S. Cabrini, E. diFabrizio, H. I. Smith and F. Stellacci, J. Am. Chem. Soc., 2005, 127, 16774–16775 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of Q-clip and NDI, ultraviolet-visible experiments, fluorescence experiments, and X-ray structure determination. See DOI: 10.1039/d0na00793e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |