Open Access Article
Open Access ArticleEnhancing FRET biosensing beyond 10 nm with photon avalanche nanoparticles†
Artur
Bednarkiewicz
 *a,
Emory M.
Chan
*a,
Emory M.
Chan
 b and
Katarzyna
Prorok
b and
Katarzyna
Prorok
 a
a
aInstitute of Low Temperature and Structure Research, Polish Academy of Sciences, Okolna 2, 50-422 Wroclaw, Poland. E-mail: a.bednarkiewicz@intibs.pl
bThe Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
First published on 18th August 2020
Abstract
Förster Resonance Energy Transfer (FRET) between donor (D) and acceptor (A) molecules is a phenomenon commonly exploited to study or visualize biological interactions at the molecular level. However, commonly used organic D and A molecules often suffer from photobleaching and spectral bleed-through, and their spectral properties hinder quantitative analysis. Lanthanide-doped upconverting nanoparticles (UCNPs) as alternative D species offer significant improvements in terms of photostability, spectral purity and background-free luminescence detection, but they bring new challenges related to multiple donor ions existing in a single large size UCNP and the need for nanoparticle biofunctionalization. Considering the relatively short Förster distance (typically below 5–7 nm), it becomes a non-trivial task to assure sufficiently strong D–A interaction, which translates directly to the sensitivity of such bio-sensors. In this work we propose a solution to these issues, which employs the photon avalanche (PA) phenomenon in lanthanide-doped materials. Using theoretical modelling, we predict that these PA systems would be highly susceptible to the presence of A and that the estimated sensitivity range extends to distances 2 to 4 times longer (i.e. 10–25 nm) than those typically found in conventional FRET systems. This promises high sensitivity, low background and spectral or temporal biosensing, and provides the basis for a radically novel approach to combine luminescence imaging and self-normalized bio-molecular interaction sensing.
Introduction
Förster Resonance Energy Transfer (FRET) is a photophysical phenomenon that enables the measurement of the colocalization and the distance between two emitting species. In biology, FRET constructs are routinely used to probe the conformation of proteins or nucleic acids, DNA hybridization, cellular membrane potential, and ligand–receptor or antibody–antigen interactions.1–4 FRET relies on resonant dipole–dipole energy transfer (ET) between a photo-excited donor (D) and a ground-state acceptor (A) molecule. For efficient ET, the donors must lie in close proximity to acceptors and overlap spectrally, i.e., the donor emission must overlap with the acceptor absorption. Various FRET detection methodologies1,5–8 and numerous D and A molecules have been exploited (e.g. fluorescent proteins, organic dyes, and fluorescent nanoparticles (NPs))1,5,9–12 so far. However, exploiting FRET between donor and acceptor molecules separated by distances larger than twice the typical Förster distance (i.e. more than 8–10 nm) remains a serious challenge. Various approaches have been proposed to address this issue, such as using triplet states of acceptors,13 localized surface plasmon Au nanoparticles as an intermediate layer between D and A,14–16 plasmonic nano-antennae,17 photonic wires18,19 and plasmonic AuNP acceptors,15,16,20 or using multiple acceptors to promote the energy transfer.21–24 Alternatively, more complex nanophotonic devices have been designed based on waveguides,25 which aim to tailor the electromagnetic field around D–A FRET constructs to enhance energy transfer and/or emission rates. The advantage of such approaches is the ability to use standard D–A fluorophore pairs; however, numerous corrections and control experiments are still required to extract quantitative and exact information, such as the D–A distance or the concentration of A, from FRET experiments.26–28 These corrections are required for reasons including the photobleaching of D or A molecules, spectral bleed-through, photoconversion or photochromism of D or A molecules, susceptibility of D and A to the local chemical environment (pH, concentration, viscosity, and aggregation) and finally sample autofluorescence, which complicates reliable and quantitative data analysis.29,30FRET donors based on lanthanide doped nanoparticles (LnNPs) offer a promising alternative to conventional donors such as fluorescent dyes, fluorescent proteins, nitrogen vacancies and quantum dots.31–33 LnNPs are used in bio-medical applications as multi-modal (e.g. fluorescence/MRI/CT contrast agents) and background free multicolour/temporal imaging labels,34–37 FRET donors in biosensors,38–41 remote optical nano-heaters42 and nano-thermometers,43,44 deep tissue NIR activatable photodynamic agents,45,46 and labels in super-resolution imaging.47,48 LnNPs show exceptional photo-stability, limited toxicity,49 long luminescence lifetimes (101 to 104 μs range), narrow-band absorption and emission at various wavelengths in the visible and near infrared regions.50–52 Moreover, the background-free detection (i.e. rejection of tissue autofluorescence) with LnNPs is of critical importance for most bio-detection/bio-imaging applications and may be achieved by time-gated and/or anti-Stokes emission.53,54 The latter method originates from the capability of lanthanide-doped upconverting nanoparticles (UCNPs) to effectively convert long wavelength photons to shorter wavelength photons (so called upconversion or UC). Although the absolute quantum efficiency of emission in such upconverting nanoparticles does not typically exceed 3%, the anti-Stokes processes in UCNPs are significantly more efficient than two-photon absorption or harmonic generation in non-linear materials.55
LnNPs have been demonstrated to be suitable for FRET antibody–antigen reaction or DNA hybridization sensing.56–58 Lanthanide based Resonance Energy Transfer (RET) with lanthanide ion donor doped inorganic nanoparticles has been demonstrated with a wide range of acceptors, such as organic dyes (e.g. rose bengal,59,60 cyanine Cy3.5,61 rhodamine B,62 a DBD-6 small organic dye63 and others64) and also with inorganic nanoparticle acceptors, such as quantum dots,9,10,65–73 gold nanoparticles74 or even lanthanides themselves.75 For the sake of clarity we will use the term FRET to denote resonant energy transfer between molecules, while RET acronyms will be used to discuss cases in which Ln3+ ions are donors. RET from LnNPs is potentially advantageous over conventional FRET because lanthanide emission produces large emission (anti)Stokes shifts and narrower emission bandwidths, which reduces background and the probability of direct photoexcitation of acceptors.
Despite these benefits, RET with LnNPs currently still suffers from insufficient sensitivity and requires further optimization. Additionally, utilizing UCNPs as RET donors is more complicated than with conventional single molecule fluorophores.76,77 Unlike for molecular FRET between two molecules, lanthanide donors Di (i.e. Di = Ln3+ ions) within a single dielectric donor nanoparticle (DNP) are capable of transferring their energy to multiple acceptors (Aj) at the NP surface in an uncorrelated manner. Moreover, this Di network is distributed randomly throughout the nanoparticle, resulting in a large distribution of Di–Aj distances. The bio-functionalization of biologically non-specific dielectric NPs with appropriate ligands as needed, e.g., antibodies attached to their surface, shifts acceptors further away from the surface of a DNP and thus donor ions become available for RET at distances larger than typical Förster distances (i.e. R0 = 6–12 nm) (Fig. 1a). Following eqn (S1),† it becomes obvious that increasing the actual D–A distance rD–A to twice the Förster distance rD–A = 2R0 reduces RET efficiency to a value as small as 1.5% of the maximum possible value (Fig. 1c), which obviously is highly disadvantageous.
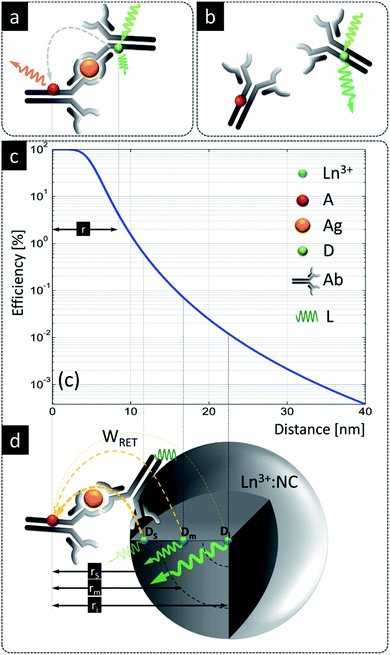 | ||
Fig. 1 A comparison of LnNPs and conventional LRET based sensing. (a) Molecular FRET between donor ( ) and acceptor ( ) and acceptor ( ) in the presence of antigen ( ) in the presence of antigen ( ); (b) lack of FRET due to missing antigen; (c) distance dependence of RET efficiency (based on eqn (S1)† for R0 = 4 nm); (d) LnNP as a RET donor contains ca. 3 zones of lanthanide donor ions, i.e. superficial (s), intermediate (m) and internal (i) donor ions ( ); (b) lack of FRET due to missing antigen; (c) distance dependence of RET efficiency (based on eqn (S1)† for R0 = 4 nm); (d) LnNP as a RET donor contains ca. 3 zones of lanthanide donor ions, i.e. superficial (s), intermediate (m) and internal (i) donor ions ( , Dx, x = s, m, i) positioned, respectively, at rs, rm and ri distances from the acceptor anchored to the surface of NPs through the Ag–Ag reaction. Ln3+ – lanthanide as the donor, D – donor, Ag – antigen, A – acceptor, Ab – antibody, L – ligand; bound (b) and unbound (d) Ag–Ab pairs using organic D and A molecules. All elements are in the scale of the X axis in (c). , Dx, x = s, m, i) positioned, respectively, at rs, rm and ri distances from the acceptor anchored to the surface of NPs through the Ag–Ag reaction. Ln3+ – lanthanide as the donor, D – donor, Ag – antigen, A – acceptor, Ab – antibody, L – ligand; bound (b) and unbound (d) Ag–Ab pairs using organic D and A molecules. All elements are in the scale of the X axis in (c). | ||
One complicating issue when using RET from LnNPs is that, due to the large number of Ln3+ dopants in a DNP and due to the large number of acceptor molecules on its surface, there are many Di–Aj pairs at variable rij distances, resulting in a wide variation of RET efficiencies (Fig. 1d). RET-active donor ions are located close to the surface (Ds), while donors located in the center of the NP (Di) do not participate in RET. Intermediate zone donors (Dm) have intermediate RET efficiencies. All three classes contribute to the total rate of energy transfer, but the presence of the latter two classes of donors (Dm, Di) reduces the sensitivity of RET-based sensors because these donors emit photons but are less susceptible to the presence of acceptors. Proposed solutions to these issues, such as decreasing the UCNP size, utilizing undoped-core/doped-shell upconverting nanoparticles59,61,66,78 or utilizing energy migration in a Gd3+ network,79 have not yet realized radical improvements in RET efficiency for ultrasensitive biosensing. Thus, there is a strong demand to better understand how the composition and architecture of a DNP affect the spectral properties and RET sensitivity of such donors. Moreover, it is important to identify alternative energy transfer mechanisms that may contribute to enhanced RET sensitivity.
In this work we theoretically predict and show that donors that host photon avalanche (PA) upconversion can enhance the interaction range over which RET can be detected, compared to conventional FRET length scales. The PA phenomenon is a highly non-linear optical process in which minute changes in excitation intensity result in many orders of magnitude changes in emission intensity critically slowing down emission risetimes.80,81 We hypothesize and confirm via modeling that the PA phenomenon should be exceptionally susceptible to any kind of perturbation such as the presence of acceptor molecules on the surface of such photon avalanche nanoparticles (ANPs). Such a sensitivity would allow ANP–acceptor pairs to operate at long distances, which would provide new opportunities for biological FRET studies.
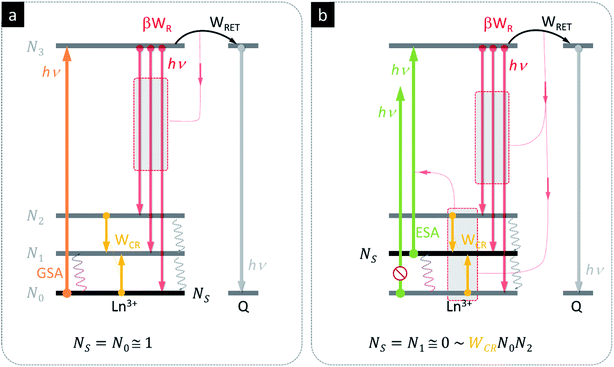
The basic photon avalanche mechanism is schematically presented in Fig. 2, where the energy levels of the same donor ion and the same acceptor are presented under two photoexcitation scenarios – ground state absorption (Fig. 2a, GSA) and excited state absorption (Fig. 2b, ESA). In both cases, the donors may either emit photons (red arrows), cross-relax (yellow arrows) or transfer energy to quencher Q (black arrows). In conventional Stokes luminescence (Fig. 2a), the absorption occurs from the ground state, whose population is close to No ≅ 1. The presence of the quencher is therefore competing only with the emission from the N3 level and ultimately, for rising Q concentration, donor emission becomes quenched, while in principle, the acceptor emission intensity rises up. The phenomena proceeding photon avalanche emission are very different (Fig. 2b), because the absorption predominantly occurs via excited state absorption (ESA). In this case, the initial population of the starting level is therefore almost empty (N1 ≅ 0); to achieve PA emission, the N1 level population must be first established. In the course of initial thermal balance with the ground state, as well as radiative (WR) or non-radiative (WCR) processes (W denotes the rate constants), this level becomes populated more and more in a non-linear way. For example, the cross-relaxation between one excited (N2) and one ground state (N0) ion doubles the population of the ESA starting level. In consequence, these phenomena result in a very steep S-shape power dependence IEMI = (IEXC)N of photon avalanche emission, with power N = 1–2 below and above the PA region, and power N = 10 (and up) above the PA threshold.
We therefore hypothesized that the strongly non-linear photophysics of PA could be exploited for efficient RET sensors, since small changes in population driven by RET would drive disproportionately larger changes in the LnNP luminescence. Bringing an acceptor molecule near a lanthanide ion donor would depopulate the first excited state (the starting level of ESA absorption) via RET, inhibiting the absorption coefficient (Fig. 2b) and disrupting the otherwise positive gain of energy looping (CR), which shall ultimately reduce the PA emission itself (Fig. 2b). We reasoned that even at large D–A distances (ηFRET < 1.5% at e.g. rDA = 2Ro), the RET might still be competitive to the recurrent population of the starting ESA level and impede PA emission. In other words, the PA based RET should not only reduce emission intensity (the result of absorbed photons), but also more importantly disrupt the positive feedback of looping and hinder the photoexcitation itself, which ultimately becomes a self-limiting phenomenon. The emission threshold of PA systems should thus be extremely sensitive to the presence of RET, because they require much higher excitation intensity to overcome losses as conventional FRET probes with linear responses.
Although photon avalanche has been successfully demonstrated in numerous lanthanide doped bulk materials,81 it is not trivial to achieve PA at room temperature or in nanomaterials intended for bio-medical purposes. Above cryogenic temperatures, the high non-linearity of PA82 is reduced due to phonon-assisted GSA and due to faster non-radiative relaxation of excited states. In nanomaterials, a proper balance must be achieved between counteracting phenomena such as cross-relaxation and energy migration (responsible for spreading the excitation over a NP). This balance is particularly challenging to maintain in small nanoparticles suspended in aqueous solutions, since energy migration also facilitates quenching of excited states at surface defects and via high energy phonons of ligands and solvents. Surface passivation with an un-doped shell can prevent surface quenching, but thick shells reduce RET efficiency by increasing the distance between the D and A. Therefore, the host composition, dopants and their concentrations as well as the architecture of NPs must be optimized83 before actual ANP nanoparticles are ready for real-world applications.
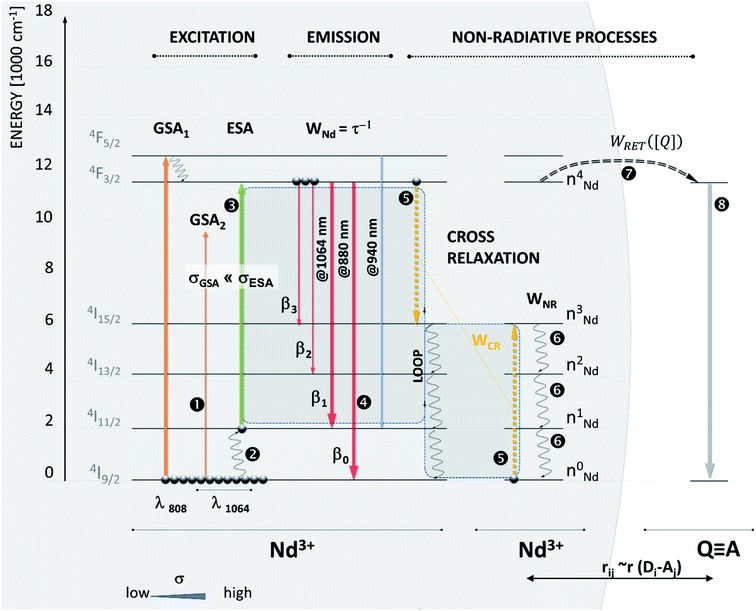
To demonstrate our Photon Avalanche Resonance Energy Transfer (PARET) approach, we computationally investigated the photophysical dynamics in the excited states of Nd3+ ions in the presence of a RET acceptor. In our model system, luminescence in these Nd3+ ions is excited by 1064 nm photoexcitation, which lies in the second NIR imaging window.84 Nd3+ doped materials are thought to exhibit PA under 1064 nm excitation since such photons, to a first approximation, are not absorbed by Nd3+ ions from the ground state due to significant, ∼2000 cm−1, energy mismatch between the incident photon energy and the gap between the excited state/ground state manifolds (Fig. 3, ➊). At physiologically relevant temperatures, the 4I11/2 level becomes thermally populated (Fig. 3, ➋) to facilitate the ESA photoexcitation of the 4F3/2 level at ∼1064 nm.
Because the acceptor depopulates the Nd3+:4F3/2 multiplet (Fig. 3, ➐), which feeds the intermediate Nd3+:4I11/2 level, the presence of the acceptor in close vicinity to ANPs and the consequent D–A RET become a competitive process to the ‘looping’ phenomenon, which is known to be a prerequisite for photon avalanche to occur. In the absence of PA, the 4F3/2 excited state population would be reduced leading to a decrease in emission intensity and luminescence lifetime shortening. However, when PA dominates the photophysics, the 4F3/2 excited state population is critically important to feed the looping phenomenon and double the population of the intermediate 4I11/2 level (Fig. 3, ➎): (4F3/2; 4I9/2) → (4I11/2; 4I11/2).
Therefore, at the PA threshold, where highly non-linear behavior is observed, changing the intermediate level population (4I11/2) a tiny bit has a disproportionately larger impact on the emission than in normal FRET systems. The presence of a quencher is thus responsible not only for quenching the 4F3/2 level population and its emission, but also for reducing the absorption coefficient (Fig. 3, ➌) and looping phenomenon (Fig. 3, ➎), which ultimately reduce the optical gain and affect the non-linear performance of ANP reporters. Hence RET sensitivity over longer distances would be expected from PA systems compared to typical fluorescence.
Because the distance- and concentration-dependent RET phenomenon, described by the term
 | (1) |
To calculate the rates of the optical transitions and energy transfer processes in Nd3+ ions, we numerically solved a set of phenomenological rate equations (see the ESI, eqn (S3)–(S8)†),82 and we evaluated them across a range of acceptor concentrations. Our PA modelling produced kinetic profiles for the populations of all Nd3+ excited states (e.g., ni from i = 0 to 4; for a detailed explanation, see Section S3†). We found that the 4F3/2 (n4) level was of critical importance since it is effectively proportional to the emission intensity at 880 nm. We quantified the steady-state intensity at a sufficiently long excitation pulse (ISS) as well as the half risetime of the emission intensity (t1/2) (as defined in Section S4, Fig. S1 and S2†). The ISS and t1/2 values were calculated as a function of D–A distance (rD–A) and as a function of the concentration of acceptor molecules (representative examples in Fig. S3†). It is intuitive that increasing the concentration of the acceptor affects the delicate balance required for PA to occur, which is evidenced (Fig. S3†) as the PA threshold and t1/2 shift as well as shifting and reducing the non-linearity of the PA phenomenon. Further, to facilitate comparisons, we defined the figure of merit as  (Fig. 4), which normalizes the steady-state ISS PA donor emission in the presence of an acceptor (quencher) (IDA) with the luminescence intensity of the donor in the absence of an acceptor (ID). For the t1/2 half-rise times, we defined a similar figure of merit as
(Fig. 4), which normalizes the steady-state ISS PA donor emission in the presence of an acceptor (quencher) (IDA) with the luminescence intensity of the donor in the absence of an acceptor (ID). For the t1/2 half-rise times, we defined a similar figure of merit as 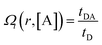 (Fig. S4d–f†).
(Fig. S4d–f†).
The figure of merit for luminescence intensity (ΩSS) was evaluated versus the distance rD–A between the D ion and A species (Fig. 4a) and at various concentrations of the acceptor [A] (Fig. 4b). Similar relationships are shown for D lifetimes (Fig. S4d–f†). Fig. 4a shows how the D emission intensity changes upon A binding at various distances. For very weak interactions (i.e. [A] = WLRET(r,[Q])/WCR = 0.001) the presence of an acceptor does not affect PA at all (ΩSS ≅ 1, pink curve). As the interaction becomes stronger, the presence of the acceptor quenches PA emission more efficiently (ΩSS ≅ 0, blue curve) at short D–A distances. In agreement with intuition, the PA luminescence intensity ‘recovers’ (i.e. ΩSS(r,[A]) approaches 1) as the A molecule is moved away from the D to distances larger than 20–30 nm (Fig. 4). The region of high slope visible in Fig. 4a is a consequence of the FRET process competing with the PA photoexcitation process. The transition region shifts to larger rD–A as the disruption of the looping (introduced by the presence and concentration of A molecules) becomes stronger. Interestingly, this means that the effective Förster distance  becomes 2–6 fold larger than the original R0 Förster distance (Fig. 4a, black curve calculated for R0 = 5 nm).
becomes 2–6 fold larger than the original R0 Förster distance (Fig. 4a, black curve calculated for R0 = 5 nm).
This increased 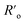 suggests that ANPs may be used to overcome the current limitations of LnNP RET donors. Specifically, ANPs can be synthesized with thicker surface passivating shells and longer linkers for bio-functionalization, while maintaining sensitivity to acceptors following the ‘spectroscopic ruler’ principle with neither additional experimental nor analytical complexity or costs. As an illustrative example, let us consider an 8 nm diameter ANP covered with a 4 nm thick un-doped shell and acceptors present 4 nm away from the surface of this donor nanoparticle. The distance between the acceptor and the center of the donor nanoparticle is thus rDA = 12 nm which conventionally would result in a very low RET efficiency (ηRET < 1%). In the PA regime, however, the WFRET being only a fraction of WCR (rDA = 12 nm, the curve indicated by the arrow in Fig. 4b) is still sufficient to make ΩSS responsive to the presence of the acceptor. This means that a single acceptor molecule could potentially ‘quench’ all dopants in an entire ANP, which is promising for biosensing. Even larger D–A distances (e.g. due to surface passivation or larger sizes of ligands and bio-recognition molecules) will impede the PA phenomenon, but then larger acceptor coverage of the ANP surface is required. We conclude that the PA system should therefore be particularly susceptible to the presence and concentration of a quencher at the surface of a PA nanoparticle.
suggests that ANPs may be used to overcome the current limitations of LnNP RET donors. Specifically, ANPs can be synthesized with thicker surface passivating shells and longer linkers for bio-functionalization, while maintaining sensitivity to acceptors following the ‘spectroscopic ruler’ principle with neither additional experimental nor analytical complexity or costs. As an illustrative example, let us consider an 8 nm diameter ANP covered with a 4 nm thick un-doped shell and acceptors present 4 nm away from the surface of this donor nanoparticle. The distance between the acceptor and the center of the donor nanoparticle is thus rDA = 12 nm which conventionally would result in a very low RET efficiency (ηRET < 1%). In the PA regime, however, the WFRET being only a fraction of WCR (rDA = 12 nm, the curve indicated by the arrow in Fig. 4b) is still sufficient to make ΩSS responsive to the presence of the acceptor. This means that a single acceptor molecule could potentially ‘quench’ all dopants in an entire ANP, which is promising for biosensing. Even larger D–A distances (e.g. due to surface passivation or larger sizes of ligands and bio-recognition molecules) will impede the PA phenomenon, but then larger acceptor coverage of the ANP surface is required. We conclude that the PA system should therefore be particularly susceptible to the presence and concentration of a quencher at the surface of a PA nanoparticle.
In conventional short range FRET in NPs, only superficial lanthanides show sufficiently strong RET to acceptors at the surface, while all other non-RET ions (i.e. the ones in the center of a NP) contribute to the overall emission and thus increase the background signal, reduce the sensitivity and increase the limit of detection. Conversely, all photon avalanche lanthanide emitters being present within the whole volume of the NPs should be capable of responding to the D–A interaction, by being quenched. Based on the results presented in Fig. 4 and S4,† we can conclude that already minute disturbances to the looping process ([A] ∼ 0.1) can change the ΩSS(r,[A]) more significantly than in conventional FRET. This is evidenced in the complete quenching of ΩSS(r,[A]) at r = 12 nm. Therefore, the monotonic responsivity of ANPs to an acceptor present at distances 2–4 fold longer than the Förster distance indicates that the PARET solves many of the currently existing drawbacks of lanthanide doped nanoparticles in bio-sensing applications.
The sensitivity of PARET systems to dedicated acceptors over long distances allows the realization of significantly brighter assays with simple technical detection setups and further miniaturization. The brightest UCNPs have passivating shells of ∼4 nm thickness, which increase the UC quantum yield by over 3 orders of magnitude.85,86 Moreover, the use of passivating shells will minimize the impact of the local chemical microenvironment87 or energy migration to surface unspecific quenchers.88 Finally, such a long-range sensitivity is also beneficial for biosensors based on LnNPs due to the finite length of ligands and linker molecules that must be attached to the surface of biologically inert LnNPs to make them biocompatible and bio-specific.
The presented example of Nd3+ ions acting as donors requires acceptors whose absorption spectra overlap with Nd3+ emission at 880 nm. Only a few NIR dyes absorb at this wavelength, but actually, the A does not need to be luminescent – gold NPs, silver NPs, or some quantum dots (e.g. Ag2S and CuInS2) with absorption bands in the NIR region should be sufficient as acceptors (or quenchers) at the required NIR spectral range. Although we use Nd3+ donors as an exemplary system, our approach of pairing PA systems with RET is general in nature, and any other PA systems at any other PA photoexcitation and PA emission wavelengths should behave in a similar manner.
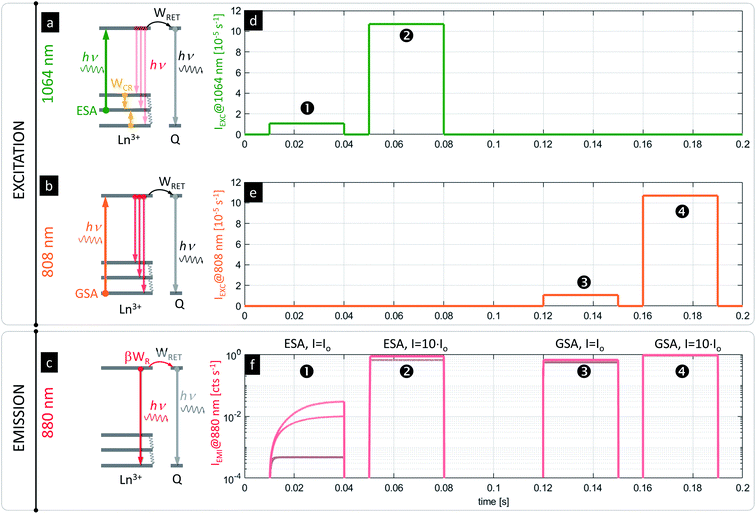
One possible concern when imaging PA nanoparticles in vitro is that the quenching of the PA emission in the presence of strong D–A interaction would make the visualization or detection of PA nanoparticles impossible. Fortunately, the advantage of the presented system is the fact that PA nanoparticles (typically meant to operate according to the scheme in Fig. 5a under 1064 nm photoexcitation pulses, Fig. 5d) can be independently excited with photons matching the GSA (e.g. at 808 nm, Fig. 5b scheme with Fig. 5e pulses) to achieve conventional luminescence (e.g. at 880 or 1064 nm, Fig. 5c and f, ➌ and ➍), which would be more efficient than ESA and PA. Because the PA phenomenon experiences saturation at certain excitation intensities above the PA threshold (Fig. 5f, ➋), the sufficiently strong (IEXC > Io) excitation at ESA wavelengths (e.g. at 1064 nm, Fig. 5d, ➋) can be concurrently used for in situ sensing (Fig. 5f, ➊) or visualization of ANPs as well (Fig. 5f, ➋) independently from RET. In fact, it could be an internal standard or a method for estimating the power absorbed by the NP, since the response would be more linear. In consequence, these features should limit the required number of negative control experiments and should simplify the data analysis or correction.
The presented modelling (Fig. 5) indicates that the same PA nanoparticles may therefore play a dual role. First, they may act as RET sensors under the PA photoexcitation regime as discussed above. At carefully adjusted excitation intensity (i.e. λEXC = 1064 nm, IEXC ∼ Io, λLUM = 880 nm for Nd3+, Fig. 5a and d), the luminescence risetimes or steady-state intensities are proportional to the concentration of acceptors (Fig. 5f, ➊ and S5†). The PA nanoparticles can additionally behave as luminescent labels, whose luminescence intensity is barely dependent on A concentration (Fig. 5f, ➋, ➌ and ➍) and is significantly stronger than in the PA sensing regime. The latter luminescence (λLUM = 880 nm) can be achieved either under “above the PA threshold” ESA photoexcitation (λEXC = 1064 nm @ e.g. IEXC > 10 × Io) (Fig. 5d, ➋) or alternatively under GSA absorption (λEXC = 808 nm) (Fig. 5b and e, ➌ and ➍). Either approach will reveal the presence of PA nanoparticles as a reference signal (in homogeneous assays or imaging in vitro) independently from bio-sensing features. Such a two-fold functionality satisfies the requirements imposed by functional FRET imaging, where D and A emission intensity images are mathematically integrated and transformed to quantify RET efficiency in order to derive the D–A interaction. This is a known issue in conventional fluorescent FRET species, which spectrally overlap with high auto-fluorescence background and light scattering in a short wavelength region (Fig. S8†). Moreover, because the emission at two different spectral bands has to be quantified (separately D and A emission), the excitation source should selectively photostimulate only donors but not acceptors, otherwise cumbersome corrections are required.1,27,89 Using the PA approach proposed here, not only a large (anti)Stokes shift is achieved, but also the same emission band is observed under two electronically switched 808 and 1064 lasers (Fig. 5a and b), or for a single 1064 nm beam with the excitation adjusted to Io and to e.g. 10 × Io (Fig. 5d, ➊ and ➋, respectively). Both wavelengths undergo low scattering and show high penetration depths into tissue (Fig. S6†), and thus are beneficial for biomedical applications. These features should possibly outperform current methods with improved sensitivity.
Further biomedical advantages of the PA nanoparticles compared to conventional organic FRET dyes or fluorescent proteins are their long, micro-to millisecond risetimes and photostable emission, which may only weakly be susceptible to the environment (e.g. viscosity and pH). These long risetimes should facilitate the rejection of any remaining, short-lived background autofluorescence, and in consequence, should significantly increase the signal-to-background ratio. Additionally, the technical convenience in recording or imaging luminescent labels with micro-to millisecond risetimes may enable PARET imaging with simple and affordable instrumentation, in contrast to the fs–ps lasers and slow TCSPC scanning lifetime imaging techniques used for organic fluorophores. Finally, we note that we have predicted82 and experimentally demonstrated that the highly non-linear behavior of the discussed ANPs enables them to be used as luminescent labels for single beam super-resolution optical imaging below the diffraction limit of light,82,83 which ultimately, all together, may enable sensing and visualization of molecular interactions at the nanoscale.
Conclusions
Retaining the photostability and NIR spectral region of operation of Nd3+ ions, the photon avalanche assisted RET (PARET) sensing modeled here should solve many drawbacks of, otherwise advantageous, LnNP donors. First, our modelling shows that depending on the concentration of acceptors on the surface of a DNP RET system, the photon avalanche emission can be quenched completely at exceptionally long D–A interaction ranges exceeding the Förster distance by a few-fold. Similar conclusions can be drawn from either steady state intensity or from luminescence risetime kinetics. There are two important consequences of this observation: (i) the surface of a DNP can be passivated with an undoped shell in order to increase its brightness and (ii) the DNP surface bio-functionalization, which typically separates acceptor molecules from donors beyond the Förster radius, would not impair its sensitivity. Additionally, the ability to photoexcite the DNPs with either GSA for conventional luminescence or with ESA for photon avalanche emission can potentially enable combining the localization of luminescent labels with their sensing capability by (a) switching between two excitation wavelengths (λGSA ∼ 808 nm, λESA ∼ 1064 nm), (b) switching the excitation intensity, or (c) switching the detection method (continuous or time-resolved decay for conventional emission vs. continuous or pulsed risetime for the photon avalanche case). These dyadic features should facilitate bio-detection and bio-functional imaging of PA probes in vitro, and thus should simplify long-term studies of biomolecular interactions and biosensing at the nanoscale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. B. acknowledges financial support from NCN, Poland, grant number UMO-2018/31/B/ST5/01827, and K. P. acknowledges financial support from NCN, Poland, grant number 2018/31/D/ST5/01328. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract No. DE-AC02-05CH11231. Part of the conceptual work was performed by A. B. in Łukasiewicz Research Network – Port Polish Center For Technology Development.References
- D. W. Piston and G. J. Kremers, Trends Biochem. Sci., 2007, 32, 407–414 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy Principles of Fluorescence Spectroscopy, 2006 Search PubMed.
- W. R. Algar, N. Hildebrandt, S. S. Vogel and I. L. Medintz, Nat. Methods, 2019, 16, 815–829 CrossRef CAS.
- X. Qiu and N. Hildebrandt, Expert Rev. Mol. Diagn., 2019, 19, 767–771 CrossRef CAS.
- B. T. Bajar, E. S. Wang, S. Zhang, M. Z. Lin and J. Chu, Sensors, 2016, 16(9), 1488 CrossRef.
- T. Zal and N. R. J. Gascoigne, Biophys. J., 2004, 86, 3923–3939 CrossRef CAS.
- I. Medintz and N. Hildebrandt, FRET - Förster Resonance Energy Transfer: From Theory to Applications, Wiley-VCH Verlag, 2013 Search PubMed.
- A. Govorov, P. L. H. Martínez and H. V. Demir, Understanding and Modeling FRETFörster-type Resonance Energy Transfer: Introduction to Förster-type Resonance Energy Transfer (FRET) FRET Series, 2016 Search PubMed.
- W. R. Algar, H. Kim, I. L. Medintz and N. Hildebrandt, Coord. Chem. Rev., 2014, 263–264, 65–85 CrossRef CAS.
- D. Geißler, S. Linden, K. Liermann, K. D. Wegner, L. J. Charbonnière and N. Hildebrandt, Inorg. Chem., 2014, 53, 1824–1838 CrossRef.
- M. Cardoso Dos Santos and N. Hildebrandt, TrAC, Trends Anal. Chem., 2016, 84, 60–71 CrossRef CAS.
- N. Hildebrandt, K. D. Wegner and W. R. Algar, Coord. Chem. Rev., 2014, 273–274, 125–138 CrossRef CAS.
- H. Hevekerl, T. Spielmann, A. Chmyrov and J. Widengren, J. Phys. Chem. B, 2011, 115, 13360–13370 CrossRef CAS.
- X. Zhang, C. A. Marocico, M. Lunz, V. A. Gerard, Y. K. Gun’Ko, V. Lesnyak, N. Gaponik, A. S. Susha, A. L. Rogach and A. L. Bradley, ACS Nano, 2014, 8, 1273–1283 CrossRef CAS.
- A. Samanta, Y. Zhou, S. Zou, H. Yan and Y. Liu, Nano Lett., 2014, 14, 5052–5057 CrossRef CAS.
- C. S. Yun, A. Javier, T. Jennings, M. Fisher, S. Hira, S. Peterson, B. Hopkins, N. O. Reich and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 3115–3119 CrossRef CAS.
- J. De Torres, M. Mivelle, S. B. Moparthi, H. Rigneault, N. F. Van Hulst, M. F. García-Parajó, E. Margeat and J. Wenger, Nano Lett., 2016, 16, 6222–6230 CrossRef CAS.
- C. M. Spillmann, S. Buckhout-White, E. Oh, E. R. Goldman, M. G. Ancon and I. L. Medintz, Chem. Commun., 2014, 50, 7246–7249 RSC.
- J. K. Hannestad, P. Sandin and B. Albinsson, J. Am. Chem. Soc., 2008, 130, 15889–15895 CrossRef CAS.
- C. Chen and N. Hildebrandt, TrAC, Trends Anal. Chem., 2020, 123, 115748 CrossRef CAS.
- Á. I. Fábián, T. Rente, J. SzölloSi, L. Matyus and A. Jenei, ChemPhysChem, 2010, 11, 3713–3721 CrossRef.
- B. P. Maliwal, S. Raut, R. Fudala, S. D'Auria, V. M. Marzullo, A. Luini, I. Gryczynski and Z. Gryczynski, J. Biomed. Opt., 2012, 17, 011006 CrossRef.
- G. Krainer, A. Hartmann and M. Schlierf, Nano Lett., 2015, 15, 5826–5829 CrossRef CAS.
- V. Raicu, J. Biol. Phys., 2007, 33, 109–127 CrossRef CAS.
- M. Baibakov, S. Patra, J. B. Claude, A. Moreau, J. Lumeau and J. Wenger, ACS Nano, 2019, 13, 8469–8480 CrossRef CAS.
- C. Berney and G. Danuser, Biophys. J., 2003, 84, 3992–4010 CrossRef CAS.
- A. Pietraszewska-Bogiel and T. W. J. Gadella, J. Microsc., 2011, 241, 111–118 CrossRef CAS.
- E. Deplazes, D. Jayatilaka and B. Corry, J. Biomed. Opt., 2012, 17, 011005 CrossRef.
- E. A. Jares-Erijman and T. M. Jovin, Nat. Biotechnol., 2003, 21, 1387–1395 CrossRef CAS.
- J. Zhang, H. Li, L. Chai, L. Zhang, J. Qu and T. Chen, J. Microsc., 2015, 257, 104–116 CrossRef CAS.
- H. H. Chen, Y.-P. Ho, X. Jiang, H.-Q. Mao, T.-H. Wang and K. W. Leong, Nano Today, 2009, 4, 125–134 CrossRef CAS.
- H. Wallrabe and A. Periasamy, Curr. Opin. Biotechnol., 2005, 16, 19–27 CrossRef CAS.
- J. Tisler, R. Reuter, A. Lämmle, F. Jelezko, G. Balasubramanian, P. R. Hemmer, F. Reinhard and J. Wrachtrup, ACS Nano, 2011, 5, 7893–7898 CrossRef CAS.
- M. E. Materia, M. Pernia Leal, M. Scotto, P. B. Balakrishnan, S. Kumar Avugadda, M. L. García-Martín, B. E. Cohen, E. M. Chan and T. Pellegrino, Bioconjugate Chem., 2017, 28, 2707–2714 CrossRef CAS.
- Y. Dai, D. Yang, D. Yu, C. Cao, Q. Wang, S. Xie, L. Shen, W. Feng and F. Li, ACS Appl. Mater. Interfaces, 2017, 9, 26674–26683 CrossRef CAS.
- M. Cardoso Dos Santos, J. Goetz, H. Bartenlian, K. L. Wong, L. J. Charbonnière and N. Hildebrandt, Bioconjugate Chem., 2018, 29, 1327–1334 CrossRef CAS.
- M. Cardoso Dos Santos, A. Runser, H. Bartenlian, A. M. Nonat, L. J. Charbonnière, A. S. Klymchenko, N. Hildebrandt and A. Reisch, Chem. Mater., 2019, 31, 4034–4041 CrossRef CAS.
- P. Alonso-Cristobal, P. Vilela, A. El-Sagheer, E. Lopez-Cabarcos, T. Brown, O. L. Muskens, J. Rubio-Retama and A. G. Kanaras, ACS Appl. Mater. Interfaces, 2015, 7, 12422–12429 CrossRef CAS.
- X. Chen, J. Wang, C. Yang, Z. Ge and H. Yang, Sens. Actuators, B, 2018, 255, 1316–1324 CrossRef CAS.
- X. Wang, S. Niazi, H. Yukun, W. Sun, S. Wu, N. Duan, X. Hun and Z. Wang, Microchim. Acta, 2017, 184, 4021–4027 CrossRef CAS.
- L. J. Charbonnière, C. Charpentier, V. Cifliku, J. Goetz, A. Nonat, C. Cheignon, M. C. Dos Santos, L. Francés-Soriano, K.-L. Wong and N. Hildebrandt, Chem.–Eur. J. DOI:10.1002/chem.202002007.
- L. Marciniak, A. Pilch, S. Arabasz, D. Jin and A. Bednarkiewicz, Nanoscale, 2017, 9, 8288–8297 RSC.
- M. D. Dramićanin, Methods Appl. Fluoresc., 2016, 4, 042001 CrossRef.
- C. D. S. D. S. Brites, A. Millán and L. D. D. Carlos, Handb. Phys. Chem. Rare Earths, 2016, 49, 339–427 CAS.
- T. M. Liu, J. Conde, T. Lipiński, A. Bednarkiewicz and C. C. Huang, Prog. Mater. Sci., 2017, 88, 89–135 CrossRef CAS.
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- Y. Liu, Y. Lu, X. Yang, X. Zheng, S. Wen, F. Wang, X. Vidal, J. Zhao, D. Liu, Z. Zhou, C. Ma, J. Zhou, J. A. Piper, P. Xi and D. Jin, Nature, 2017, 543, 229–233 CrossRef CAS.
- Q. Zhan, H. Liu, B. Wang, Q. Wu, R. Pu, C. Zhou, B. Huang, X. Peng, H. Ågren and S. He, Nat. Commun., 2017, 8, 1058 CrossRef.
- A. Gnach, T. Lipinski, A. Bednarkiewicz, J. Rybka and J. A. Capobianco, Chem. Soc. Rev., 2015, 44, 1561–1584 RSC.
- D. J. Naczynski, M. C. Tan, M. Zevon, B. Wall, J. Kohl, A. Kulesa, S. Chen, C. M. Roth, R. E. Riman and P. V. Moghe, Nat. Commun., 2013, 4, 2199 CrossRef CAS.
- H. Dong, S.-R. R. Du, X.-Y. Y. Zheng, G.-M. M. Lyu, L.-D. D. Sun, L.-D. D. Li, P.-Z. Z. Zhang, C. Zhang and C.-H. H. Yan, Chem. Rev., 2015, 115, 10725–10815 CrossRef CAS.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS.
- D. Casanova, D. Giaume, T. Gacoin, J. P. Boilot and A. Alexandrou, J. Phys. Chem. B, 2006, 110, 19264–19270 CrossRef CAS.
- J. M. Zwier and N. Hildebrandt, Time-gated FRET detection for multiplexed biosensing, in Reviews in Fluorescence 2016, ed. C. D. Geddes, Springer, Cham, 2017, pp. 17–43 Search PubMed.
- D. J. Gargas, E. M. Chan, A. D. Ostrowski, S. Aloni, M. V. P. Altoe, E. S. Barnard, B. Sanii, J. J. Urban, D. J. Milliron, B. E. Cohen and P. J. Schuck, Nat. Nanotechnol., 2014, 9, 300–305 CrossRef CAS.
- K. Kuningas, T. Ukonaho, H. Pakkila, T. Rantanen, J. Rosenberg, T. Lovgren and T. Soukka, Anal. Chem., 2006, 78, 4690–4696 CrossRef CAS.
- T. Soukka, T. Rantanen and K. Kuningas, Ann. N. Y. Acad. Sci., 2008, 1130, 188–200 CrossRef CAS.
- Y. H. Wang, P. Shen, C. Li, Y. H. Wang, Z. H. Liu, Z. J. Wu and Z. H. Liu, Anal. Chem., 2013, 85, 258–264 CrossRef CAS.
- Y. Wang, K. Liu, X. Liu, K. Dohnalová, T. Gregorkiewicz, X. Kong, M. C. G. Aalders, W. J. Buma and H. Zhang, J. Phys. Chem. Lett., 2011, 2, 2083–2088 CrossRef CAS.
- C. D. Laboda and C. L. Dwyer, Adv. Funct. Mater., 2016, 26, 2866–2874 CrossRef CAS.
- S. Bhuckory, E. Hemmer, Y.-T. Wu, A. Yahia-Ammar, F. Vetrone and N. Hildebrandt, Eur. J. Inorg. Chem., 2017, 2017, 5186–5195 CrossRef CAS.
- Y. Ding, F. Wu, Y. Zhang, X. Liu, E. M. L. D. de Jong, T. Gregorkiewicz, X. Hong, Y. Liu, M. C. G. Aalders, W. J. Buma and H. Zhang, J. Phys. Chem. Lett., 2015, 6, 2518–2523 CrossRef CAS.
- A. L. De Guereñu, P. Bastian, P. Wessig, L. John and M. U. Kumke, Biosensors, 2019, 9, 9 CrossRef.
- L. Francés-Soriano, N. Peruffo, M. M. Natile and N. Hildebrandt, Analyst, 2020, 145, 2543–2553 RSC.
- H. Liu, J. Han, C. McBean, C. S. Lewis, P. Kumar Routh, M. Cotlet and S. S. Wong, Phys. Chem. Chem. Phys., 2017, 19, 2153–2167 RSC.
- S. Melle, O. G. Calderón, M. Laurenti, D. Mendez-Gonzalez, A. Egatz-Gomez, E. Lopez-Cabarcos, E. Cabrera-Granado, E. Diaz and J. Rubio-Retama, J. Phys. Chem. C, 2018, 122(32), 18751–18758 CrossRef CAS.
- T. Cheng, R. Marin, A. Skripka and F. Vetrone, J. Am. Chem. Soc., 2018, 140, 12890–12899 CrossRef CAS.
- A. P. Litvin, P. S. Parfenov, E. V. Ushakova, T. A. Vorsina, A. L. Simões Gamboa, A. V. Fedorov and A. V. Baranov, J. Phys. Chem. C, 2015, 119, 17016–17022 CrossRef CAS.
- S. Cui, S. Xu, H. Song, W. Xu, X. Chen, D. Zhou, Z. Yin and W. Han, RSC Adv., 2015, 5, 99099–99106 RSC.
- L. Mattsson, K. D. Wegner, N. Hildebrandt and T. Soukka, RSC Adv., 2015, 5, 13270–13277 RSC.
- Y.-W. Li, L. Dong, C.-X. Huang, Y.-C. Guo, X.-Z. Yang, Y.-J. Xu and H.-S. Qian, RSC Adv., 2016, 6, 54241–54248 RSC.
- F. Zhang, C.-L. Zhang, W.-N. Wang, H.-P. Cong and H.-S. Qian, ChemSusChem, 2016, 9, 1449–1454 CrossRef CAS.
- Y. Wang, B. Si, S. Lu, E. Liu, X. Hu and J. Fan, Sens. Actuators, B, 2017, 246, 127–135 CrossRef CAS.
- Y. Zhang, G. Wang, L. Yang, F. Wang and A. Liu, Coord. Chem. Rev., 2018, 370, 1–21 CrossRef CAS.
- L. Labrador-Páez, D. J. Jovanović, M. I. Marqués, K. Smits, S. D. Dolić, F. Jaque, H. E. Stanley, M. D. Dramićanin, J. García-Solé, P. Haro-González and D. Jaque, Small, 2017, 13, 1700968 CrossRef.
- T. Forster, Naturwissenschaften, 1946, 33, 166–175 CrossRef CAS.
- T. Förster, Ann. Phys., 1948, 437, 55–75 CrossRef.
- D. Wawrzynczyk, M. Nyk, A. Bednarkiewicz, W. Strek and M. Samoc, J. Lumin., 2013, 133, 138–144 CrossRef CAS.
- R. Deng, J. Wang, R. Chen, W. Huang and X. Liu, J. Am. Chem. Soc., 2016, 138, 15972–15979 CrossRef CAS.
- M. F. Joubert, S. Guy and B. Jacquier, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 10031–10037 CrossRef CAS.
- M.-F. Joubert, Opt. Mater., 1999, 11, 181–203 CrossRef CAS.
- A. Bednarkiewicz, E. M.-Y. Chan, A. M. Kotulska, L. Marciniak and K. Prorok, Nanoscale Horiz., 2019, 4, 881–889 RSC.
- C. Lee, E. Xu, Y. Liu, A. Teitelboim, K. Yao, A. Fernandez-Bravo, A. Kotulska, S. H. Nam, Y. D. Suh, A. Bednarkiewicz, B. E. Cohen, E. M. Chan and P. J. Schuck, arxiv:2007.10551.
- E. S. Levy, C. A. Tajon, T. S. Bischof, J. Iafrati, A. Fernandez-Bravo, D. J. Garfield, M. Chamanzar, M. M. Maharbiz, V. S. Sohal, P. J. Schuck, B. E. Cohen and E. M. Chan, ACS Nano, 2016, 10, 8423–8433 CrossRef CAS.
- C. Würth, S. Fischer, B. Grauel, A. P. Alivisatos and U. Resch-Genger, J. Am. Chem. Soc., 2018, 140, 4922–4928 CrossRef.
- X. Xu, Z. Zhou, Y. Liu, S. Wen, Z. Guo, L. Gao and F. Wang, APL Photonics, 2019, 4, 026104 CrossRef.
- S. Fischer, N. D. Bronstein, J. K. Swabeck, E. M. Chan and A. P. Alivisatos, Nano Lett., 2016, 16, 7241–7247 CrossRef CAS.
- N. J. J. J. Johnson, S. He, S. Diao, E. M. Chan, H. Dai and A. Almutairi, J. Am. Chem. Soc., 2017, 139, 3275–3282 CrossRef CAS.
- R. Roy, S. Hohng and T. Ha, Nat. Methods, 2008, 5, 507–516 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00404a |
| This journal is © The Royal Society of Chemistry 2020 |