Open Access Article
Open Access ArticleIdentification of nanomaterials by the volume specific surface area (VSSA) criterion: application to powder mixes†
Claire
Dazon
 a,
Vanessa
Fierro
a,
Vanessa
Fierro
 b,
Alain
Celzard
b,
Alain
Celzard
 b and
Olivier
Witschger
b and
Olivier
Witschger
 *c
*c
aLaboratoire de Physique et de Métrologie des Aérosols, Institut de Radioprotection et de Sûreté Nucléaire, F-91192 Gif-sur-Yvette, France
bInstitut Jean Lamour, UMR CNRS 7198, F-88051, Epinal, France
cLaboratoire de Métrologie des Aérosols, Institut National de Recherche et de Sécurité, F-54519 Vandœuvre les Nancy, France. E-mail: olivier.witschger@inrs.f
First published on 15th September 2020
Abstract
We demonstrate the relevance of the Volume Specific Surface Area (VSSA) parameter to identify the nanoparticulate character of powder mixes based on either spherical constituent particles with bimodal size distributions (TiO2), or fiber-like constituent particles with unimodal size distributions (sepiolite and sepiolite-based pigments). These new results indicate that VSSA could reasonably be proposed as an optional criterion in the future for the definition of nanomaterials based on the European Commission recommendation, provided certain requirements are fulfilled.
Introduction
Nowadays, nanomaterials integrate numerous products of our daily life.1 In particular, food, agriculture and the pharmaceutical industry are the sectors where the consumption of nanoparticles is the highest.1–3 The French R-Nano report for nanomaterial declaration4 stated that powders are clearly the most encountered materials in research and industry. For instance, carbon black, silica, calcium carbonate, titanium dioxide and boehmite (AlO(OH)) are the five most produced, imported or distributed nanomaterials declared in France, and this trend seems to be also verified in the rest of Europe, where similar documents are proposed.5 In general, the declared nanoparticles in these documents can be assumed as powders, even if their nanoparticulate nature is almost never indicated.The current questioning on the potential harmful effects of nanoparticles on health and environment leads to a crucial need to develop sustainably these materials recognised for their amazing properties at the nanoscale. Safe-by-design approach as well as risk assessment in workplaces where nanoparticles (especially powders) are handled are the first relevant methodologies to this objective.6,7 However, they require that nanomaterials can be identified from a chosen definition, which will depend on the context in which the nanoparticulate powders are used.8 The European Commission (EC) definition recommendation9 is the most practical to implement, and covers a broad range of research and industrial areas. For example, the European REACH regulation recently proposed an appendix for nanoforms applicable to the guidance on registration and identification of substances.10,11 There has been a significant increase in the number of research projects, around twenty in 2020, to improve the identification of nanomaterials for any situation encountered in industry and in research fields concerning health, environment, risk assessment, sustainable development of nanomaterials, etc. OECD has proposed since 2006 a global forum for discussion of nano-safety issues and a test guidelines program is currently underway in this view.12
Currently, the most recent guide document on the implementation of the definition of nanomaterials proposed by the EC is the report of the Joint Research Centre (JRC)13 in which the last advances on this topic are taken into account. According to this report, the median number size distribution based on the external dimensions of the constituent particles, and the fraction smaller than 100 nm should be assessed through a reference measurement system to identify nanomaterials. It gives detailed examples of measurement techniques for this, with their pros and cons. Electron Microscopy (EM) techniques (TEM or SEM) appear to be the most reliable methods to confirm or deny a nanoparticulate nature based on the number size distribution.
However, according to the same document, it is also possible to use the Volume Specific Surface Area (VSSA) criterion as a proxy to facilitate nanomaterial identification, provided certain requirements are fulfilled. The VSSA (eqn (1))13 is indeed more operational than an EM method since it requires determining the material's external specific surface area AEx and its skeletal density ρ, which are respectively obtained by gas adsorption and gas pycnometry following adapted protocols. These latter are largely documented in the literature, relatively well accessible in laboratories and industries, and are especially suitable for powder analysis. This approach also reduces possible artefacts we can have for EM techniques such as the operator influence during image analysis and the particle counting for instance.
| VSSA (m2 cm−3) = AEx (m2 g−1) × ρ (g cm−3) | (1) |
Recent studies have shown that the VSSA can be used beyond the EC definition and can be applied to identify non-nanomaterial powders.14–16 Nevertheless, the VSSA criterion is more likely to lead to this false negative result since it is an integral approach (the average equivalent particle size can be deduced from the VSSA13). Given the many benefits that VSSA can offer for the identification of nanomaterials, especially for small professional structures which cannot afford to spend too much time and invest in sophisticated techniques, the VSSA must be further studied. This suggests that much more experimental data should be produced on a variety of representative industrial powders, including non-nanomaterial powders, which has hardly been done except for the above mentioned studies.14–16 It is important to note that the majority of the materials studied under the VSSA criterion in these works are relatively monodisperse, non-porous and of pure chemistry (a single compound for the constituent particles). However, powder mixes consisting of two or more populations of particles of unique or different shapes and chemical compositions have not been treated to date even though they are frequently encountered in workplaces. In these particular cases, EM methods may be questioned regarding to the relevant identification of nanomaterials since no clear procedures are proposed. Based on the existent literature, two approaches may be applied for such powder mixes cases:
• Case 1: one may consider each of the populations separately, perform the characterization for each of them, and determine the percentage of particles < 100 nm for each of the 2 observed populations,
• Case 2: on the images, it is not possible to distinguish 2 populations of particles, so, in this case, one may treat the observed particles as belonging to a single population and proceed as in the case of a single powder, with the: determination of the percentage of particles < 100 nm for all observed particles.
What is the criterion for choosing case 1 or case 2 if the nanomaterial nature of this powder mix is to be determined? Both approaches involve an obvious operator artifact relying on individual judgment and skills in absence of stabilized protocols. In such a situation, the VSSA approach is interesting because it implements a unique documented procedure that has been described for a long time and frequently updated.
In this context, in order to continue and strengthen the demonstration of the VSSA relevancy for nanomaterial identification, we present herein a comparison between the average equivalent particle sizes determined by VSSA and SEM on powder mixes, applying the operational methodology developed in a previous work on this subject.15 These materials are characterised either by unimodal, fibre-like constituent particles (sepiolite and composite pigments) or by bimodal spherical constituent particles (mixes of nano and non-nano TiO2). These types of powders have never been studied before in this context and constitute a new and original data set on complex materials required today to clearly demonstrate the relevance of VSSA for the identification of nanomaterials.
Materials & methods
Powder studied
We studied 12 powders among these 9 are powder mixes. A powder mix corresponds, in our study, to two types of pure materials of the same chemical nature that have been mixed to obtain a powder maintaining a priori some characteristics of the two original powders. Table 1 presents the materials we have studied with their expected or theoretical AEx and skeletal densities. The theoretical values of these parameters were calculated by a linear weighted relation of those obtained for pure TiO2 A and TiO2 E. The complete characterisation data of these two powders are available in the literature.16,17 However, the raw number particle size distributions of these pure TiO2 powders are given in ESI (Fig. S3 and S4 respectively)† in order to complete our discussion of the results presented below for the characterization of powder mixes by the SEM method. The median diameters for the constituent particles are 138 nm (fraction < 100 nm = 11%) and 7 nm (fraction < 100 nm = 100%) for TiO2 A and TiO2 E respectively. The series of TiO2 mixes was produced manually in the laboratory, based on a sampling process17 of pure TiO2 A and TiO2 E (of close spherical particle shape).| Powder | Code (proportion in the mix, in wt%) | Expected AEXa, m2 g−1 | Expected ρb, g cm−3 |
|---|---|---|---|
| a The expected AEx are based on the combination of AEx of TiO2 A (9.5 m2 g−1) and AEx TiO2 E (142 m2 g−1) with the weight fractions chosen. For sepiolite and sepiolite-based pigments, the indicated range of AEx is given by the manufacturer. b TiO2 A and TiO2 E are pure anatase powders and have the same expected skeletal density of 3.92 g cm−3. Whatever the weight fractions used, the skeletal density will be similar for all the TiO2 mixes, and there should not be any change in the value since the anatase structure is maintained. For sepiolite, the expected skeletal density is based on the manufacturer's technical data sheet and is close to the skeletal density of silica (2.20 g cm−3). | |||
| TiO2 | TiO2 A/TiO2 E (90/10) | 46.0 | 3.92 |
| TiO2 A/TiO2 E (80/20) | 82.0 | ||
| TiO2 A/TiO2 E (70/30) | 118.0 | ||
| TiO2 A/TiO2 E (60/40) | 154.5 | ||
| TiO2 A/TiO2 E (50/50) | 191.0 | ||
| Sepiolite | Sepiolite | 320–340 | 2.00 |
| Sepiolite based pigment | B19 | ||
| BN 19 | |||
| R10 | |||
| J4 | |||
Sepiolite (a mineral clay of chemical formula Si12Mg8O30(OH)4(OH2)4·8H2O; characterised by porous fibre-like particles), and the 4 pigments (composite materials based on sepiolite fibres in which molecules of organic dye were inserted inside the tunnels), were purchased from Pigm'Azur (France). These four pigments are called mixes because they are synthesised from the combination of two powders (pure sepiolite and a powder dye) to obtain one. More information on the structure of sepiolite and pigments is available in the ESI† and in the literature.18–22 Note that pigments are among the most produced, imported and distributed materials in France,4 between 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons.
000 tons.
Powder characterisation
The powder mixes were characterised according to the methodology explained in detail elsewhere.16 Briefly, the powders were observed by SEM to determine the number-based size distributions of the constituent particles (see Table S1 in ESI† for SEM sample preparation and SEM parameters). For each case, between 200 and 400 particles were counted randomly through a total of 30 SEM images. We measured the equivalent diameter of the projected area (when spherical particles are observed) or the smallest dimension (in the case of fibers) to determine the number size distribution and the fraction < 100 nm (noted fN). The approach we have adopted for SEM images is case 2 we discussed in the Introduction section where we consider only one particle population for counting. This choice relies on the “worst” case, where the operator is not experienced in clearly distinguishing between two particle populations according to their shape, for instance. This approach is also easier to achieve in a relative short time.To determine the VSSAs, measurements of surface areas were carried out by nitrogen adsorption with application of BET and t-plot methods. For t-plot analysis, we used the Harkin–Jura thickness curve for the N2 molecule,23 adapted to the case of metal oxides. Indeed, only a fraction of the total surface area of microporous powders corresponds to their external area, which is involved in the EC nanomaterial definition. Thus, applying the BET method to microporous powders leads to an area ABET much higher than AEx because, unlike the t-plot method, the BET cannot distinguish between the internal surface area developed by micropores and the solid external area. Therefore, BET application to microporous powders increases the probability of false positives. Besides, the literature lacks a comparison between BET and t-plot methods for the determination of VSSA and their impact on the correct identification of nanomaterial or non-nanomaterial powders. Consequently, we have chosen to use both BET and t-plot methods to determine ABET and AEx, respectively, for VSSAs and compare the results. Determining the VSSAs also requires knowing the skeletal densities, which were determined by helium pycnometry. The experimental conditions for outgassing prior to nitrogen adsorption and helium pycnometry measurements are described in the ESI (Table S2).† The material is considered nanoparticulate if its VSSA is higher than 60 m2 cm−3. Below 20 m2 cm−3, the powder is not considered a nanomaterial. Between 20 and 60 m2 cm−3, the SEM method is used to classify definitely the powder in the non-nanomaterials or nanomaterials category. The powder classifications by VSSA and SEM are then compared through the equivalent average particle size to evidence false positives or false negatives by VSSA.
Results and discussions
Gas adsorption, helium pycnometry and VSSA results
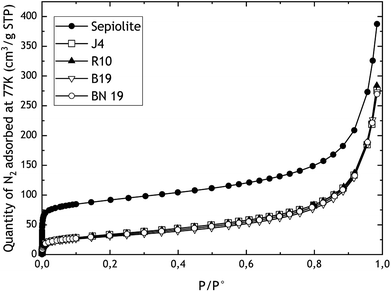
Fig. 1 and 2 show the nitrogen adsorption isotherms obtained for TiO2 mixes and sepiolite and pigments, respectively. On Fig. 1, we display also the absorption isotherms of pure TiO2 A and TiO2 E taken from literature.17Table 2 summarises the values of ABET and AEx, obtained after application of BET and t-plot models, respectively, and skeletal density to finally calculate the VSSA and classify the powders in or out of the nanomaterials category.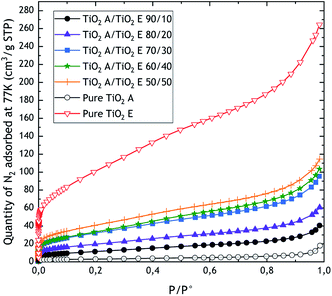 | ||
| Fig. 1 Adsorption isotherms of the TiO2 powder mixes. The data for pure TiO2 A and TiO2 E were taken from literature.17 | ||
| Powder | A BET (BET) m2 g−1 | A Ex (t-plot) m2 g−1 | ρ g cm−3 | Average VSSA m2 cm−3 | Nano VSSA? |
|---|---|---|---|---|---|
| a The indicated values are averages based on three gas adsorption measurements for each powder sample. In the case of BET, the area is not necessarily external, which is the case of powders based on porous materials. Hence the name ABET instead of AEx. The uncertainties given are standard deviations indicating the experimental error, the latter including the accuracy of the measuring tool and human error. b The indicated values of skeletal density are average values for TiO2 powders, but for sepiolite and pigments, it was not possible to repeat the measurements. The uncertainty indicated corresponds to the uncertainty given by the manufacturer for one measurement with the Accupyc 1340 pycnometer. | |||||
| TiO2 A/TiO2 E (90/10) | 41.2 ± 0.6 | 41.9 ± 0.6 | 3.81 ± 0.01 | 157 ± 16 (BET) | Yes |
| TiO2 A/TiO2 E (80/20) | 66.0 ± 5.0 | 66.5 ± 0.8 | 3.75 ± 0.03 | 247 ± 45 (BET) | Yes |
| TiO2 A/TiO2 E (70/30) | 99.0 ± 15.0 | 99.5 ± 14.0 | 3.73 ± 0.01 | 368 ± 77 (BET) | Yes |
| TiO2 A/TiO2 E (60/40) | 121.5 ± 1.6 | 120.0 ± 1.2 | 3.67 ± 0.01 | 445 ± 26 (BET) | Yes |
| TiO2 A/TiO2 E (50/50) | 143.0 ± 2.5 | 141.0 ± 1.5 | 3.64 ± 0.01 | 520 ± 31 (BET) | Yes |
| Sepiolite | 342.0 ± 2.0 | 151.0 ± 1.0 | 2.45 ± 0.01 | 369 ± 2 (t-plot) | Yes |
| B19 | 109.0 ± 1.0 | 104.0 ± 1.0 | 2.30 ± 0.01 | 248 ± 3 (BET) | Yes |
| BN 19 | 116.0 ± 0.5 | 114.0 ± 2.0 | 2.28 ± 0.01 | 265 ± 2 (BET) | Yes |
| R10 | 124.0 ± 1.0 | 120.0 ± 1.0 | 2.30 ± 0.01 | 284 ± 1.5 (BET) | Yes |
| J4 | 123.0 ± 0.5 | 121.0 ± 1.0 | 2.33 ± 0.01 | 285 ± 1.5 (BET) | Yes |
In the case of TiO2 mixes, we can observe that the microporosity increases with the proportion of TiO2 E, as expected. In fact, TiO2 E is characterised by a mixed adsorption isotherm (type I(b) + II) highlighting microporosity, and therefore high surface area.16,17 Indeed, the type I isotherm is characterized by a high uptake of nitrogen at very low relative pressures (P/P° < 0.01) resulting from the filling of ultramicropores (pore diameter under 0.7 nm). Since the micropores are totally filled (P/P° < 0.2), and if the material does not present pore diameters wider than 2–3 nm, the material does not adsorb a significant amount of gas until the capillary condensation occurs, at around P/P° = 1. The type I(b) isotherm is related to a microporous materials with a developed pore size distribution including wide micropores and possibly narrow mesopores (<2.5 nm). The raw TiO2 A is characterised by a type II isotherm, characteristic of a nonporous or macroporous solid24 The shape is the result of unrestricted monolayer–multilayer adsorption up to capillary condensation. When we qualify an isotherm as mixed type, this means that it combines two of the types of isotherms given by the IUPAC classification. This is the case for the TiO2 E where we can observe a very high nitrogen uptake at very low relative pressures, followed by a progressive adsorption until capillary condensation. Consequently, the more the TiO2 E is present, the higher the microporosity, which is given by TiO2 E. As with conventional type I isotherms, those of TiO2 mixes exhibit a rapid increase in N2 uptake at relative pressures less than 0.1. Nevertheless, the comparison of ABET and AEx measured by BET and t-plot methods, respectively, shows that there is no significant differences between the two approaches, which means that the increase of microporosity for TiO2 mixes is very limited. The relative difference between values from BET and t-plot is comprised between −1.4% and 1.7% with respect to the BET. Finally, based on the weight fractions in the TiO2 mixes, we obtained biases ranging from −20% to −9% on the external surface area. For skeletal densities, we obtained values close to expectations (average bias of 2% in absolute value).
The raw adsorption isotherms of sepiolite and pigments are type II, with microporosity evidenced in the case of raw sepiolite. Indeed, for sepiolite, the ABET is 55% higher than AEx measured by t-plot. Moreover, the quantity of N2 adsorbed at very low relative pressure is indicative of the presence of micro-pores. But for pigments, there are no significant differences between the two approaches, the values of AEx by the t-plot method being just 1.6 to 4% lower than ABET. Besides, we do not observe differences between pigments in terms of adsorption isotherms and therefore surface area. However, a marked change in the sepiolite surface area can be observed in the presence of dyes. Indeed, when dyes are mixed with raw sepiolite, as explained by Ovarlez et al.,19,20 the dye is inserted into the channels of the sepiolite fibres, thus filling the microporosity. These results also show that the outgassing protocol, applied to sepiolite and pigments, was relevant since we preserved the powder particle surfaces (no intrinsic desorption of water from sepiolite or dye degradation for pigments). Indeed, differential thermogravimetric measurements have shown that the zeolitic water of sepiolite is removed between 35 and 200 °C, the first coordinated water between 120 and 365 °C, the second coordinated water between 365 and 600 °C and the structural water between 600 and 950 °C, whereas the dyes begin to be degraded at 300 °C.20 Knowing that the materials were outgassed at room temperature prior to our nitrogen adsorption experiments, we considered that the whole structure of the sepiolite and the pigments was preserved. No gas adsorption data are available in the literature for the sepiolite and sepiolite based pigments we purchased. However for the raw sepiolite, it is possible to compare our results with those of Fitaroni et al.,25 where sepiolite was used to offer several possibilities for the production of organically modified materials for different applications. In their study, pure sepiolite has a 296 m2 g−1ABET, which is quite close to our value, confirming that our results are coherent with those obtained elsewhere.
The results obtained here show that for non-porous powders, the surface areas obtained by the BET or t-plot method are equivalent. Knowing that BET is the best known and the easiest to access for non-specialists in the field of material characterisation, it can be systematically proposed for the determination of VSSA, provided that the corresponding isotherm clearly indicates that the material is intrinsically non-porous.
By applying the VSSA calculation (eqn (1)), all powders are classified as nanomaterials according to the official EC recommendation since their VSSA values are higher than 60 m2 cm−3. At this stage of the powder characterisation, the materials can be correctly identified as nanomaterials by VSSA or constitute false positives. If we want to confirm this classification by the number size distribution reference parameter, we should observe more than 50% of the particles above the size threshold of 100 nm. False positives are not as serious as false negatives. Indeed, if we recall certain contexts for which the identification of nanomaterials is crucial, for instance occupational exposure or environmental contamination, a false positive would entail taking additional measures to ensure safety with regard to nano-powders incorrectly identified as such by VSSA. Reports from the EC13 and different studies in the open literature14–16 have stated that a VSSA value above 60 m2 cm−3 may be sufficient to classify a powder in the nanomaterials category without supplementary characterisation. We can also prove that in the case of sepiolite, for which we also applied the BET model for the determination of AEx (taking into account the microporosity), there is no influence on the final nanomaterial identification since the VSSA value for the sepiolite with the BET model leads to the same conclusion. The following paragraphs will present the results of the SEM characterisation of the powder mixes and the comparison with the VSSA.
SEM results and comparison of the classification of powder mixes with VSSA
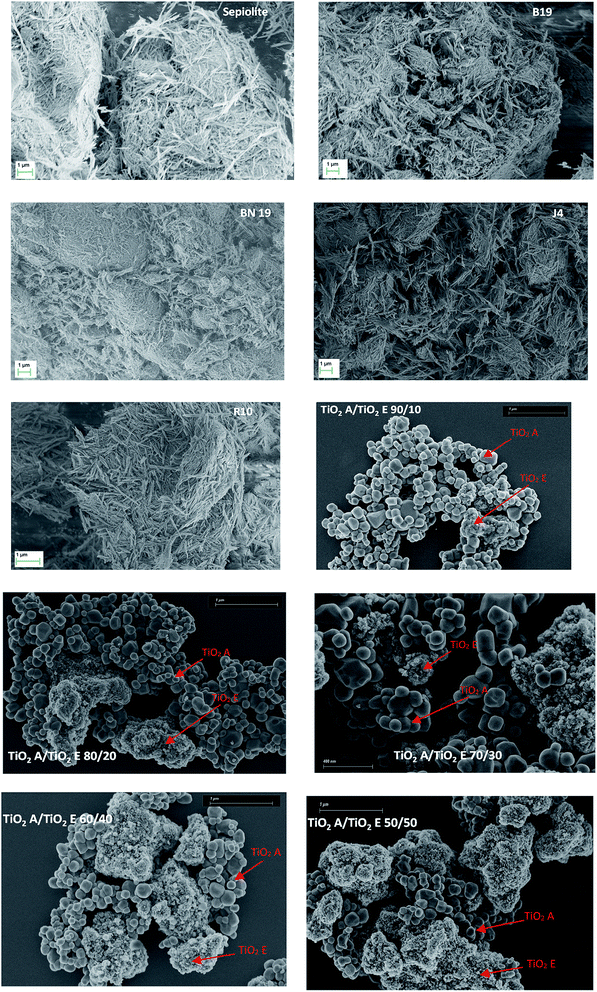
Fig. 3 presents typical SEM images obtained for the different powder mixes. The corresponding number size distributions are available in the ESI (Fig. S5 for TiO2 mixes and Fig. S6 for sepiolite and pigments).†Table 3 summarises the SEM results in terms of the number of particles counted (N), the median sizes of the constituent particle D50 deduced from the number size distributions, the minimum Dpp,min and maximum Dpp,max equivalent projected area diameter (sphere case) or the lowest dimension (in the case of the fibers) and the fraction fN smaller than 100 nm.| Powder | N (−) | D 50 (nm) | D pp,min (nm) | D pp,max (nm) | f N < 100 nm (%) | Nano SEM? |
|---|---|---|---|---|---|---|
| TiO2 A/TiO2 E (90/10) | 234 | 124 ± 15 | 3 | 353 | 29 | No |
| TiO2 A/TiO2 E (80/20) | 203 | 77 ± 11 | 4 | 211 | 63 | Yes |
| TiO2 A/TiO2 E (70/30) | 353 | 87 ± 5 | 3 | 340 | 54 | Yes |
| TiO2 A/TiO2 E (60/40) | 463 | 26 ± 7 | 3 | 313 | 79 | Yes |
| TiO2 A/TiO2 E (50/50) | 369 | 23 ± 7 | 9 | 366 | 70 | Yes |
| Sepiolite | 192 | 73 ± 2 | 11 | 183 | 84 | Yes |
| B19 | 305 | 83 ± 1.5 | 40 | 193 | 72 | Yes |
| BN 19 | 404 | 56 ± 1.5 | 18 | 124 | 97 | Yes |
| R10 | 280 | 45 ± 1.5 | 16 | 163 | 96 | Yes |
| J4 | 230 | 51 ± 2 | 24 | 192 | 95 | Yes |
As expected, the TiO2 powder mixes present two populations of constituent particles, one typical of pure TiO2 A (D50 of 138 nm) and the other typical of pure TiO2 E (D50 of 7 nm). The agglomerates of TiO2 E are all the more present as the mix is enriched with this powder. The constituent particles of the two powders are assumed to be spherical, based on the SEM images obtained (the spherical shape is not visible in Fig. 3 for TiO2 E, but other SEM micrographs at higher magnification allowed us to observe a spherical shape). Previous studies16,17 have also demonstrated this particle characteristic from TEM observations of TiO2 E. The results of particle counting have not revealed any clear difference between TiO2 A and TiO2 E in terms of populations proportions for mixes 90/10, 80/20 and 70/30. However, the 60/40 and 50/50 mixes show a predominance of TiO2 E in terms of number of constituent particles inside the mixes as observed on typical SEM images.
It was confirmed that the constituent particles of sepiolite and pigments were fibre-like, in good agreement with the literature.18,19 The unimodal size distributions obtained after particle counting show a median diameter between 45 and 83 nm, which is consistent with the literature data indicating a fibre diameter between 20 and 30 nm for raw sepiolite,20 if we take into account the uncertainties. Indeed, we have found larger fibres. Note that powders based on sepiolite are characterised by a clear polydispersity and that the length of the fibres is 5 μm on average. Based on the number size distributions, sepiolite and pigments are classified in the category of nanomaterials, which is in accordance with the VSSA classification.
If we now compare the VSSA and SEM classifications, we can observe that only one mix is not correctly identified by VSSA (TiO2 A/TiO2 E 90/10). This powder mix is not a nanomaterial according to the particle size distribution of the SEM number (more than 50% of the particles have a median diameter above the 100 nm threshold, with a fN of 29%), whereas the VSSA identifies this powder as a nanomaterial. We therefore have a false positive case. Note, however that this mix is composed of a non-negligible fraction of nanoparticle. Indeed, we can suppose that, in absence of clear indications on how we should count the particles detected on the SEM micrographs, it is likely that an fN value above 50% could have been obtained. Regarding to the size reference criterion, the TiO2 A/TiO2 E 90/10 is a false negative by SEM and a true nanomaterial powder with the VSSA. This example demonstrates the added value of VSSA in such powder mix case. The unique outgassing protocol associated to a common gas adsorption and isotherm data treatment (BET method) allows unambiguous identification of nanomaterials.
Fig. 4 is a parity plot comparing the average equivalent particle sizes obtained by VSSA and SEM. This representation does not allow the two populations of TiO2 mixes to be observed since average sizes are displayed, but this graph aims at highlighting the agreement between the VSSA approach in terms of nanomaterial classifications with respect to a reference method.
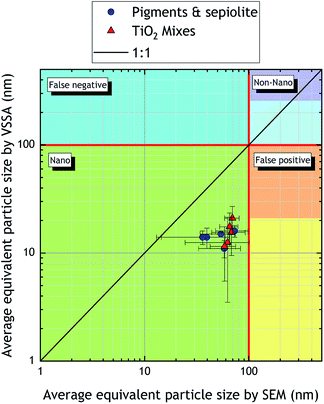 | ||
| Fig. 4 Parity plot comparing average equivalent particle sizes obtained with VSSA and SEM for the powder mixes studied in this work. The error bars indicate standard deviations. | ||
We can see that even if the average equivalent particle size by VSSA is systematically different (by more than 50% in absolute value) from that obtained by SEM, considering SEM uncertainties reduces this discrepancy (between −10% to −20%). The discrepancies observed, i.e., a systematic underestimation of the equivalent particle size by VSSA compared to SEM is quite surprising since the opposite is more expected. This is because the mass of particles increases generally faster than their surface-area, and VSSA is inversely proportional to the particle diameter. Therefore, for a polydisperse material such as industrial powders, the size value will be lower than the one of a monodisperse material with the same average diameter. Measuring a VSSA below a selected threshold value does not necessarily indicate that particles are greater than 100 nm. By extension, the equivalent diameter based on the VSSA might be falsely overestimated and lead to false negative. Here, our equivalent diameters based on the VSSA seem to be underestimated compared to the reference SEM method. This could be partly explained by the great influence of the operator's appreciation when measuring the projected area, particularly when the powders are highly polydispersed. Indeed, as mentioned it in the introduction section, there are no clear rules to manually measuring the projected area with SEM, and maybe, based on the results obtained on these powder mixes, the reference method for particle size determination could be reversed since the VSSA is less influenced by operator artifact.
We can see that no false positive or false negative is obtained through this comparison. The VSSA alone could have been sufficient to classify the powders as nanomaterials. Given the external surface areas measured, the probability of obtaining a false negative was very low. Indeed, a false negative case would have been very likely if the VSSA measured in this study had been close to the thresholds of 60 m2 cm−3 for TiO2 or 40 m2 cm−3 for sepiolite and pigments, if we take into account the particle morphology (spherical particles for TiO2, fibre-like particles for sepiolite and pigments) as explained in a previous document.13 Here, the VSSAs are much higher, more than 50% than the different thresholds. Following the approach of Dazon et al.16 where the particle morphology is not taken into account during the VSSA classification, a false negative would also have been improbable (the lower limit used is 20 m2 cm−3). The results obtained here also suggest that the VSSA parameter can be used both with the BET model and with the t-plot, without causing any change in the classification into nanomaterials or non-nanomaterials. Indeed, in the case of sepiolite for which we have evidenced microporosity, the use of BET or t-plot models to determine the external specific surface area leads to the same nanomaterial classification.
On the one hand, systematic use of the BET model can increase the probability of false positive classification due to potential overestimation of the AEx induced by microporosity. Since false positive cases are less serious than false negatives with regard to risk assessment for environment or occupational health, it seems prudent to promote the use of the BET model for the classification of nanomaterials if we choose to base it only on VSSA.
On the other hand, in the case of microporous powders, the internal surface area can prevail over the external surface area, and the powder can be incorrectly classified as a nanomaterial, thus entailing overprotective safety measures for its handling.
Such false nanomaterial identification could have an impact on the competitive development of the product. Thus, in order to optimise the use of VSSA for nanomaterial identification, it would be interesting to indicate clearly the context involved.
It seems obvious that for risk assessment, it is necessary to limit false negatives as much as possible and therefore to propose to apply only the BET model for the determination of surface area. In this case, there is no distinction between external surface and internal surface (if present in the powder). In other sectors such as industry, a proper identification of nanomaterials or non-nanomaterials can be crucial, and a clear distinction between external and internal surface area for VSSA determination must be made. In this particular context, the t-plot model could be proposed for the VSSA determination instead of the BET model. If the powder is only characterised by an external surface (no micropores), the t-plot or BET model will lead to the same value. It can be however emphasised that the BET model is much better known than the t-plot. It would therefore be worth proposing, in an industrial problem, to determine the surface area with the BET model for any given type of gas adsorption isotherms (non-porous, mesoporous or microporous), then, depending on the surface area measured, apply the t-plot model to distinguish the external surface from the internal surface. We could propose for instance a BET surface area threshold for which we are sure that a t-plot application would lead to the same classification as with BET. For example, for highly microporous powders such as charcoal or activated carbon, the surface areas are generally higher than 1000 m2 g−1. In this case, the t-plot model is necessary to distinguish clearly the external surface from the internal surface in order to classify properly the powder in the category of nanomaterials or not. If we obtain a surface area between 300 and 500 m2 g−1, the microporosity is less pronounced and applying the t-plot will lead to the same result as the BET model.
This suggestion can be illustrated through the study on single wall carbon nanotubes (SWCNTs) carried out by Ansón et al.26 In their study, the SWCNTs, synthesized by arc-discharge method, have an ABET of 265 m2 g−1 and an AEx of 105 m2 g−1. The skeletal density (obtained by helium pycnometry) is 2.08 g cm−3. When we determine the VSSA with ABET, we obtain 551 m2 cm−3 and 218 m2 cm−3 when using AEx. In both cases, the SWCNTs are identified as nanomaterials based on the VSSA values, which is an expected result, even though we do not have the particle size or shape. It is important to consider here that the microporous nature of the SWCNTs is relatively small and the gap between the ABET and AEx is not enough to prefer AEx in the VSSA calculation rather than ABET. The 300–500 m2 g−1 threshold for ABET that we propose is suitable here for the rapid identification of SWCNTs as nanomaterial based solely on surface area by the BET method (the simplest and most accessible) and skeletal density measurements. However, it is clear that for the future, studies should be carried out on this point in order to determine critical surface area values for which the BET method cannot be relevant for the determination of VSSA.
Finally, these new results encourage further VSSA investigations to generalise its use for the classification of nanomaterials without confirmation by a number size distribution. Indeed, we have presented powder mixes with bimodal number size distributions for which one could have been falsely identified as a non-nanomaterial due to lack of clear and relevant EM procedures for particle counting in such powder mix situation. Moreover, the comparison shows that the bimodal or the unimodal character does not seem to have any influence on the correct classification into the nanomaterial category via VSSA and using the conservative approach.16 This statement can extend to the particle morphology since we had spherical or fibre-like particles. Here, this parameter could be reasonably used as an optional parameter for nanomaterial classification and not as a proxy, at least for spherical and fibre-like particles, when the shape is already known. However, the main issue now requiring further studies is the tolerance that we can offer for the different thresholds related to VSSA and nanomaterial classification. Here, the VSSA obtained are far above the thresholds, giving more confidence in the VSSA classification, but the case of boundary powders is still difficult. It could be envisaged in the future to study powders with VSSA close to the threshold of 20 m2 cm−3 and characterised by different particle morphologies and polydispersities.
Conclusion
This study brings new results of the relevance of the Volume Specific Surface Area (VSSA) criterion applied to 9 powder mixes (bimodal TiO2 mixes, sepiolite and sepiolite-based pigments) for the identification of nanomaterials. We showed that for the determination of the VSSA, which requires the knowledge of external specific surface area and skeletal density, the values of surface area can be indifferently determined by the BET or t-plot method in the case of non porous powders (differences of less than 2% between the two models in absolute value). If the materials are (micro)porous, which can be easily evidenced from their N2 adsorption isotherms, the t-plot method should be preferred, otherwise there is a significant risk of obtaining false positives. By a simple comparison with the SEM method, we show that 8 mixes of powders composed of organic–inorganic particles (4 pigments) with a fibre-like constituent particle shape or nano/non-nano TiO2 (4 powder mixes) with sphere-like constituent particles are identified by VSSA as nanomaterial powders, which is confirmed by the SEM reference method. It is interesting to note that although the comparison of the average equivalent diameter determined by the SEM and VSSA methods shows discrepancies, the final results for nanomaterial identification by VSSA is correct, suggesting that VSSA could be used as a single parameter for nanomaterial identification. Moreover, although the difference is relatively small when using BET or t-plot models for non porous powders, it is necessary to choose carefully in case of microporous materials. The choice between the two methods for a relevant VSSA determination has never been clearly explained in literature and could hamper its practical use.Currently, the open literature is still lacking of sufficient experimental data to prove the relevance of using VSSA as an optional criterion without risk of false identification. This promising criterion is obviously more attractive for small companies that need to identify nanomaterials in the workplace in a safe-by-design or risk assessment context. Indeed, it is more operational with regard to the techniques to be implemented (nitrogen adsorption and helium pycnometry) than the reference method of electron microscopy. Moreover, the lack of EM procedures to adequately address the case of powder mixes may lead to a misinterpretation of the nanomaterial or non-nanomaterial nature of the substance, due to the important operator artefact in this case. Unfortunately, in absence of several examples of reliable identification non-nanomaterials with VSSA and no false negative obtained, this criterion should be still used as a surrogate pending new experimental data. For the future, several ideas can be developed:
• Focus on the specific case of clays that are the most probable false negative nanomaterial with the VSSA parameter. Indeed, clays materials are fiber-like (as sepiolite) or platelets-like particles and their external specific surface area is generally low (<20 m2 g−1),
• Need to include other non-nanomaterial powders in future studies to support the results related to the appropriate use of VSSA as a single criterion for nanomaterial identification without the risk of false negatives,
• More work should also be done on microporous materials, as this type of powder is the most likely false positive,
• Conduct studies on thresholds related to the use of t-plot model for the VSSA determination, indeed, external surface area limits for which the probability of false positive and false negative are extremely low could be proposed on the basis of significant experimental data. This could increase the use of the VSSA as the sole criterion to identify a nanomaterial or a non-nanomaterial. Special recommendations should also be associated, in particular on the outgassing conditions for adsorption and pycnometry measurements on which the VSSA is based,
• Evaluate the different metrics and assess the most relevant ones (mass metrics, surface metrics) for nanomaterial identification,
• Finally, for all the points aforementioned, a diversity of particle shapes should be integrated.
Nomenclature
| A Ex (m2 g−1) | External specific surface area determined with the t-plot method |
| A BET (m2 g−1) | Total specific surface area determined with the BET method, equivalent to AEx in absence of micropores |
| D 50 (nm) | Median size of the constituent particles |
| D pp (nm) | Constituent particle size |
| D pp,max (nm) | Maximum constituent particle size measured |
| D pp,min (nm) | Minimum constituent particle size measured |
| f N (%) | Fraction of constituent particles lower than 100 nm |
| N (−) | Number of constituent particles counted on SEM images |
| ρ (g cm−3) | Skeletal density determined with helium pycnometry |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to thank Sandrine Mathieu from Institut Jean Lamour (CC-MEM) for giving us high-quality SEM images of TiO2 powder mixes within a short time. We also thank Mathieu Pinault and Vincent Mertens from the CEA Saclay for providing us with the Ultra 55 Zeiss for observing sepiolite and pigments. Finally, we thank Nicolas Volle for delivering sepiolite and pigments in a reduced time and for all the advices and discussions concerning the characterisation results.References
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella Jr, D. Rejeski and M. S. Hull, J. Nanotechnol., 2015, 6, 1769–1780 Search PubMed.
- R. J. B. Peters, H. Bouwmeester, S. Gottardo, V. Amenta, M. Arena, P. Brandhoff, H. J. P. Marvin, A. Mech, F. B. Moniz, L. Q. Pesudo, H. Rauscher, R. Schoonjans, A. K. Undas, M. V. Vettori, S. Weigel and K. Aschberger, Trends Food Sci. Technol., 2016, 54, 155–164 Search PubMed.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, J. Nanopart. Res., 2012, 14, 1109 Search PubMed.
- Ministère de la Transition Ecologique et Solidaire, https://www.ecologique-solidaire.gouv.fr/sites/default/files/2018-12%20-%20Rapport%20R-nano%202018.pdf.pdf, accessed March 2020.
- European Chemical Agency, https://euon.echa.europa.eu/fr/national-reporting-schemes, accessed March 2020.
- Y. Ding, T. A. J. Kuhlbusch, M. Van Tongeren, A. Sánchez Jiménez, I. Tuinman and R. Chen, et al. , J. Hazard. Mater., 2017, 322, 17–28 Search PubMed.
- K. H. Dunn, A. C. Eastlake, M. Story and E. D. Kuempel, Ann. Work Exposures Health, 2018, 62, 362–388 Search PubMed.
- X. Gao and G. V. Lowry, NanoImpact, 2018, 9, 14–30 Search PubMed.
- EC, Commission recommendation of 18 October 2011 on the definition of nanomaterial, Official Journal of European Union, 2011, vol. L275, pp. 49–62 Search PubMed.
- European Chemical Agency, https://echa.europa.eu/documents/10162/13655/how_to_register_nano_en.pdf/f8c046ec-f60b-4349-492b-e915fd9e3ca0, accessed March 2020.
- L. P. W. Clausen and S. F. Hausen, Nat. Nanotechnol., 2018, 13, 766–768 Search PubMed.
- K. Rasmussen, H. Rauscher, P. Kearns, M. González and J. R. Sintes, Regul. Toxicol. Pharmacol., 2019, 104, 74–83 Search PubMed.
- H. Rauscher, et al., Identification of nanomaterials through measurements, EUR 29942 EN, Publications Office of the European Union, Luxembourg, 2019, DOI:10.2760/053982, ISBN 978-92-1037, JRC118158.
- W. Wohlleben, J. Mielke, A. Bianchin, A. Ghanem, H. Freiberger, H. Rauscher, M. Gemeinert and V.-D. Hodoroaba, J. Nanopart. Res., 2017, 19, 61 Search PubMed.
- C. Dazon, O. Witschger, S. Bau, V. Fierro and P. L. Llewellyn, Environ. Sci.: Nano, 2019, 6, 152–162 Search PubMed.
- C. Dazon, O. Witschger, S. Bau, V. Fierro and P. L. Llewellyn, Nanoscale Adv., 2019, 1, 3232–3242 Search PubMed.
- C. Dazon, PhD thesis, Aix-Marseille University, 2019.
- F. Giulieri, S. Ovarlez and A. M. Chaze, Int. J. Nanotechnol., 2011, 8, 10–12 Search PubMed.
- S. Ovarlez, F. Giulieri, F. Delamare, N. Sbirrazzuoli and A. M. Chaze, Microporous Mesoporous Mater., 2011, 8142, 371–380 Search PubMed.
- S. Ovarlez, F. Giulieri, A. M. Chaze, F. Delamare, J. Raya and J. Hirschinger, Chem.–Eur. J., 2009, 15, 11326–11332 Search PubMed.
- J. Raya, J. Hirschinger, S. Ovarlez, F. Giulieri, A. M. Chaze and F. Delamare, Phys. Chem. Chem. Phys., 2010, 12, 14508–14514 Search PubMed.
- S. Ovarlez, A. M. Chaze, F. Giulieri and F. Delamare, C. R. Chim., 2006, 9, 1243–1248 Search PubMed.
- W. D. Harkins and G. Jura, J. Am. Chem. Soc., 1944, 66(8), 1362–1366 Search PubMed.
- M. Thommes, K. Kaneko, V. Neimark Alexander, P. Olivier James, F. Rodriguez-Reinoso, J. Rouquerol and S. W. Sing Kenneth, Pure Appl. Chem., 2015, 87, 1051 Search PubMed.
- L. B. Fitaroni, T. Venâncio, F. H. Tanaka, J. C. F. Gimenez, J. A. S. Costa and S. A. Cruz, Appl. Clay Sci., 2019, 179, 105149 Search PubMed.
- A. Ansón, M. A. Callejas, A. M. Benito, W. K. Maser, M. T. Izquierdo, B. Rubio, J. Jagiello, M. Thommes, J. B. Parra and M. T. Martínez, Carbon, 2019, 4, 1243–1248 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00395f |
| This journal is © The Royal Society of Chemistry 2020 |