Open Access Article
Open Access ArticleCancer treatment by magneto-mechanical effect of particles, a review
Cécile
Naud
ab,
Caroline
Thébault
a,
Marie
Carrière
 c,
Yanxia
Hou
c,
Yanxia
Hou
 c,
Robert
Morel
a,
François
Berger
b,
Bernard
Diény
c,
Robert
Morel
a,
François
Berger
b,
Bernard
Diény
 a and
Hélène
Joisten
a and
Hélène
Joisten
 *ad
*ad
aUniv. Grenoble Alpes, CEA, CNRS, Spintec, 38000 Grenoble, France. E-mail: helene.joisten@cea.fr
bBrainTech Lab, U1205, INSERM, Univ. Grenoble Alpes, CHU-Grenoble, France
cUniv. Grenoble Alpes, CEA, CNRS, IRIG-SyMMES, 38000 Grenoble, France
dUniv. Grenoble Alpes, CEA, LETI, 38000 Grenoble, France
First published on 19th June 2020
Abstract
Cancer treatment by magneto-mechanical effect of particles (TMMEP) is a growing field of research. The principle of this technique is to apply a mechanical force on cancer cells in order to destroy them thanks to magnetic particles vibrations. For this purpose, magnetic particles are injected in the tumor or exposed to cancer cells and a low-frequency alternating magnetic field is applied. This therapeutic approach is quite new and a wide range of treatment parameters are explored to date, as described in the literature. This review explains the principle of the technique, summarizes the parameters used by the different groups and reports the main in vitro and in vivo results.
1. Introduction
Magnetic nano or micro particles have very attractive properties for biomedical applications.1,2 So far, they have been widely studied and/or used for magnetic resonance imaging (MRI) as contrast agents,3,4 magnetic targeting in particular for drug delivery5,6 and sometimes in combination with MRI,7 as part of the theranostics concept,8 regenerative medicine,9 cell sorting,10 tissue engineering,11 protein purification,12 and for hyperthermia.13–15Magnetic nano or microparticles are nano or micrometric sized materials with particular magnetic properties, which allow them to be remotely operated thanks to an externally applied magnetic field. They have a large surface-to-volume ratio, which is favorable to graft a large number of molecules onto their surface. It also promotes their interaction with biological entities such as cells, viruses, proteins and DNA.16 Moreover, their magnetic properties can be modulated thanks to the shape and the composition of constitutive materials, so that they can be mechanically actuated, attracted to a region of interest, rotated, or used to generate a local heating. This is particularly interesting in the context of the expanding field of biomechanics, where they can be used to apply local forces or torques on biological specimens and to study the cellular response. Beside and in synergy with the classical modulation of molecular pathways using molecular medicine and/or targeted therapy, magneto-mechanical strategies should pave the way for new therapeutical strategies in the field of cancer. Targeted therapies modulate specifically specific molecular pathways involved in cancer such as proliferation, angiogenesis, cell death, invasion or immunosuppression for example. This has been a major biomedical progress. However, several limitations are emerging such as molecular resistances and relapse through molecular adaptation for heterogeneous tumor formations. Accessibility to the tumor microenvironment is also a major limitation. Magneto-mechanical therapies will not address a specific molecular target but will implement a specific physical action inside the tumor micro-environment providing new therapeutical opportunities. This new field of physics and medicine and biology will support a major inter-disciplinary work that is enlighten in this review.
The first uses of magnetic nanoparticles subjected to low-frequency alternating magnetic field to obtain a mechanical action on cells date back to 2008. Super Paramagnetic Iron-Oxide Nanoparticles (SPIONs) have been used to modify the intracellular calcium concentration of mast cells17 or to activate the mechanosensitive TREK-1 receptor18 involved in neuroprotection, epilepsy and depression, among others. In the same period, the interest of magnetic particles with shape anisotropy emerged, with the use of nickel nanowires to reduce the viability of fibroblasts.19
Considering the effects induced by low-frequency movements of magnetic particles and the possible mechanical effects of particles currently designed for hyperthermia,20,21 a new technique to destroy cancer cells by low-frequency mechanical vibrations of magnetic particles was proposed and demonstrated in 2010.22 The pioneering interdisciplinary study – involving nanomagnetism and biology – of Kim et al., 2010,22 likewise presented in the chapter of Novosad and Rozhkova, 2011,23 highlighted this remarkable phenomenon of cancer cells destruction through the mechanical vibration of bio-functionalized magnetic vortex structures, which may lead, in the longer term, to potential treatment of cancer with few sides effects. For the first time, a non-thermic mechanical force demonstrate its therapeutical effect. This promising approach gradually expanded over the last decade, as presented here. The most recent literature includes in particular the review of Goiriena-Goikoetxea et al., 2020,24 showing disk-shaped magnetic particles as magneto-mechanical actuator to destroy cancer cells, Chen et al., 2020,25 showing a magneto-mechanical approach based on nanocubes considered as nanospinner, and Maniotis et al., 2019,26 showing an alternating magnetic field source designed for the magneto-mechanical activation of particles. These articles emphasize the topicality and growing interest in this magneto-mechanical approach. In the present review, the principle of the treatment by magneto-mechanical effect of particles is explored. The different parameters which could be modulated are summarized, as well as the main in vitro and in vivo results presented in the literature. The perspectives for renewing our medical efficacy in cancer therapy are also summarized.
2. Presentation of cancer treatment by magneto-mechanical effect of particles
2.1 Principle
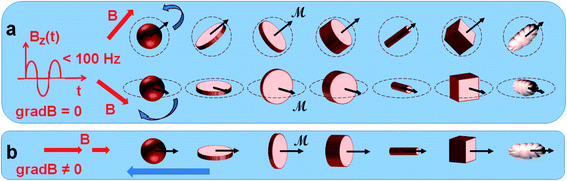
This method aims at inducing the cancer cell death or tumor destruction, by means of low-frequency mechanical vibrations of magnetic particles, with a very localized effect and thereby preserving the neighboring healthy cells. The induction of physical properties responding to the specific pattern of cancer cells and tissue is also an explanation for the specificity and safety of this approach. Like for chemotherapies or targeted therapies, the specific physics deregulation of cancer cells would explain the devoted effect of specific mechanical parameters on cancer cells. This relatively recent technique requires multidisciplinary expertise. In the literature, a multitude of terms can be found for it, including magnetolysis, magneto-mechanical actuation or stimulation therapy, magneto-actuation, non-chemo-toxic induction of cell death, mechanical tumor destruction, non-temperature induced effects, vibration or oscillations of magnetic particles by the application of a non-heating field that can be rotary or alternating at low frequency. This makes the review of the literature quite complex. For the rest of this article, we have chosen to use the term treatment by magneto-mechanical effect of particles (TMMEP), which refers both to the magnetic source of the stimulus and to the mechanical response of the particles that will act on cells.Fundamental principle of the magneto-mechanical effect: the physical principle of the magneto-mechanical effect involved in “TMMEP” lies in the magnetic particles ability to be remotely actuated by an external magnetic field, in various configurations, as sketched in Fig. 1 and ref. 24 and 27. In an applied magnetic field B considered as uniform over the entire volume of the particle, the average magnetic moment ![[scr M, script letter M]](https://www.rsc.org/images/entities/b_char_e145.gif) of the particle, which itself depends on the amplitude and direction of the applied field B, is subjected to the magnetic torque
of the particle, which itself depends on the amplitude and direction of the applied field B, is subjected to the magnetic torque ![[scr M, script letter M]](https://www.rsc.org/images/entities/b_char_e145.gif) × B, and thus tends to align with the direction of the field. Meanwhile, if the magnetic anisotropy of the particle is high enough (exhibiting strong particle-composition, size, and shape dependence), the direction of the magnetic moment
× B, and thus tends to align with the direction of the field. Meanwhile, if the magnetic anisotropy of the particle is high enough (exhibiting strong particle-composition, size, and shape dependence), the direction of the magnetic moment ![[scr M, script letter M]](https://www.rsc.org/images/entities/b_char_e145.gif) remains almost blocked within the particle, parallel – or making a small angle – with the axis known as the easy axis of magnetization, or maintained in the easy plane of magnetization. On particles released or partially anchored in fluidic solutions, the effect of the magnetic torque becomes magneto-mechanical. It tends to re-orient the particle itself, until its easy axis or easy plane align with the applied magnetic field direction (like the earth's magnetic field effect on a compass needle). Rotating – or more generally variable – magnetic fields, spatially uniform, are thus used to induce continuous rotation or vibration of the particles in TMMEP. In this approach requiring effective magnetic torque, highly anisotropic particles are often preferred, such as for instance magnetic disks with “magnetic shape anisotropy”,24 or with “perpendicular magnetic anisotropy”.27
remains almost blocked within the particle, parallel – or making a small angle – with the axis known as the easy axis of magnetization, or maintained in the easy plane of magnetization. On particles released or partially anchored in fluidic solutions, the effect of the magnetic torque becomes magneto-mechanical. It tends to re-orient the particle itself, until its easy axis or easy plane align with the applied magnetic field direction (like the earth's magnetic field effect on a compass needle). Rotating – or more generally variable – magnetic fields, spatially uniform, are thus used to induce continuous rotation or vibration of the particles in TMMEP. In this approach requiring effective magnetic torque, highly anisotropic particles are often preferred, such as for instance magnetic disks with “magnetic shape anisotropy”,24 or with “perpendicular magnetic anisotropy”.27
Subjected to the variable magnetic field, the particle thus transfers its mechanical energy to its environment, on biological cells, in fluids or tissues. The frequency of the particle rotation or vibration is however limited to a few tens of Hz, the amplitude of mechanical vibrations decreasing sharply at higher frequencies owing to the viscosity of the fluid. The physical properties of the targeted tissue microenvironment will also impact the therapeutical response.
Furthermore, the magnetic forces generated on particles by a non-uniform field (i.e. through non-zero magnetic field gradient)26 tend to move the particles in translation towards the regions of large field amplitudes. These forces can potentially be used to bring the particles towards targeted regions within a living organism.28 Less effective in this approach than the torque effect, the gradient of a non-uniform magnetic field can likewise be used for generating the particles oscillations, through temporal variations of the field-gradient.29 Particles being firstly rotated by the magnetic torque as a function of the field direction, the non-zero magnetic field gradient then guides the motion of particles.26,28
2.2 TMMEP parameters
For TMMEP, parameters such as shape, size and magnetic properties of the particles, frequency and amplitude of the applied magnetic field must be chosen in order to maximize the mechanical response of the particles within the biological tissue. As this technique is quite new and studied by groups with different backgrounds, there is a diversity in the parameters chosen by the different teams. A synthetic overview of the particle shapes, dimensions, compositions and magnetic properties, is presented in Fig. 2. Indeed, the type of particles and their functionalization, the applied field and the type of cancer cells greatly differ from one article to another, showing the extent of possibilities. Table 1 list the main parameters used in these studies, highlighting their heterogeneity. | ||
| Fig. 2 Representative scheme showing properties of particles with different shapes, associated sizes and materials, and obtained results: microscopy images of particles and magnetization curves (magnetization as a function of magnetic field). Extracted from: (a and b) Shen et al., 2017 (ref. 30) with (a) magnetization curve of dry particles at 300 K and (b) TEM images of iron oxide particles doped with zinc (l = 62 nm) [Reproducted with permission (ref. 30), Copyright© 2017, Ivyspring International Publisher, Theranostics]. (c) Kilinc et al., 2015:34 SEM image of Fe–Au nanorods (d = 254 nm and l = 2 μm) [Reproducted with permission (ref. 34), Copyright© 2015, Wiley-VCH, Adv. Healthcare Mater.]. (d) Martínez et al., 2016:35 SEM image of Fe nanowire (l = 6.4 ± 1.3 μm and d = 30–40 nm) [Reproducted with permission (ref. 35), Copyright© 2016, Springer Nature, Sci. Rep.]. (e) Contreras et al., 2015:33 magnetization loops of an array of Ni nanowires (l = 4 μm and d = 30–40 nm) with magnetic field applied in the in-plane (black) and out-of-plane (red) directions [Reproducted with permission (ref. 33), Copyright© 2015, Dove Press, Int. J. Nanomed.]. (f and g) Wong et al., 2017 (ref. 36) with (f) hysteresis loop of NiFe particles with d = 150–350 nm (black to blue curve, respectively) and l = 500 nm, and (g) SEM images of NiFe particles of d = 350 nm and l = 75 nm, 200 nm and 500 nm (from left to right on the image) [Reproducted with permission (ref. 36), Copyright© 2017, Springer Nature, Sci. Rep.]. (h) Leulmi et al., 2015:38 SEM image of NiFe particles (d = 1.3 μm and l = 60 nm) [Reproducted with permission (ref. 38), Copyright© 2015, Royal Society of Chemistry, Nanoscale]. (i) Mansell et al., 2017:27 out-of-plane (red) and in-plane (black) hysteresis loops (b) for an array of 2 μm CoFeB/Pt particles and (d) for an array of 2 μm NiFe vortex particles [Reproducted with permission (ref. 27), Copyright© 2017, Springer Nature, Sci. Rep.]. (j) D. Cheng et al., 2014:32 TEM image of iron oxide particles (d = 200 ± 50 nm) [Reproducted with permission (ref. 32), Copyright© 2014, Springer, Nanoscale Res. Lett.]. (k) Wo et al., 2016:45 magnetization curve of hollow magnetic nanospheres of Fe3O4 (d = 250–550 nm) [Reproducted with permission (ref. 45), Copyright© 2016, Ivyspring International Publisher, Theranostics]. (l and m) Chiriac et al., 2018 (ref. 52) with (l) SEM image and (m) magnetization curve of Fe68.2Cr11.5Nb0.3B20 particles (l = 10–200 nm) [Reproducted with permission (ref. 52), Copyright© 2018, Springer Nature, Sci. Rep.]. | ||
| Shape | References | Diameter | Length or thick-ness | Material | Functionalization | Chemo | Device | Field | Amplitude | Frequency | Duration | Cell line | Type1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a The § symbol is used to indicate in vivo studies; abbreviations: EGF: epidermal growth factor; Oscillat. = Oscillating; Grad. = Gradient; Alternat. = Alternating; Sinus. = Sinusoidal. b In the last column (“Type”) of the present Table 1, A refers to “Apoptosis” mentioned as main cell death pathway, post-TMMEP, reported from the column 6 of Table 2. c 1 Human cells, except mentioned. 2 Zn–IO: iron oxide doped with zinc. 3 Triphenyl-phosphonium cation. 4 5% metallic impurities. 5 Cervical cancer metastasis. 6 Vemurafenib. 7 Doxorubicin. 8 Synthetic antiferromagnet (SAF) composed of: Au/(Ta/Pt/CoFeB/Pt/Ru/Pt/CoFeB/Pt)/Au. 9 Mouse Ehlrich ascite adenocarcinoma. 10 Silica shell + graphene quantum dots + lipid bilayer. 11 Contamination by HeLa cells was recently demonstrated.10312 Cetuximab. | |||||||||||||
| Cube | Shen et al., 2017 (ref. 30) | — | 62 nm | Zn–IO2 | DA–PAA–PEG + EGF peptide | — | Magnetic stirrer | Rotating | 40 mT | 15 Hz | 30 min × 3 days | U87 | Glioblastoma A |
| Chen et al., 2020 (ref. 25) | — | 20 nm | Zn–IO2 | TPP3 | — | Magnetic stirrer | Rotating | 40 mT | 15 Hz | 30 min | U87 | Glioblastoma A | |
| Nanowire | Fung et al., 2008 (ref. 19) | 200 nm | 4.4 μm | Ni | — | — | Magnetic stirrer | Rotating | 240 mT | 1 Hz | 20 min | NIH/3T3 | Fibroblast |
| Liu et al., 2012 (ref. 31) | 100 nm | 1 μm | C+4 | — | — | Magnetic stirrer | Rotating | 40–75 mT | 16.7 Hz | 20 min | MCF-7 | Breast cancer | |
| Wang et al., 2013 (ref. 29) | 80 nm | 580 nm | Fe | Silica | — | Oscillating magnet | Oscillat. or Grad. | 160 kA m−1 ∼ 200 mT | 2–10 Hz | 20–60 min | HepG2 | Hepatocellular carcinoma | |
| D. Cheng et al., 2014 (ref. 32) | 250–120 nm | 200 nm | Fe3O4 | — | — | Electro-magnet | Alternat. | 35 kHz | 0–10–30–60–120 min | HeLa5 | Metastases A | ||
| Contreras et al., 2015 (ref. 33) | 35 nm | 4 μm | Ni | — | — | Coil | Alternat. | 0.5 mT | 1–1000 Hz | 10–30 min | HCT116 | Colorectal carcinoma | |
| Kilinc et al., 2015 (ref. 34) | 254 nm | 1.98 μm | Fe–Au | PEG + HRG | Yes6 | Electro-magnet | Alternat. + Grad. | — | 0.5 Hz (1 s ON + 1 s OFF) | 15 min | MCF7, MDA-MB-231 | Breast cancer | |
| Martínez et al., 2016 (ref. 35) | 30–40 nm | 6.4 μm | Fe | BSA, APTES | Yes7 | Electro-magnet | Alternat. | 1 mT | 10 Hz | 10 min | MDA-MB-231 | Breast cancer | |
| Cylinder | Wong et al., 2017 (ref. 36) | 150–350 nm | 50–500 nm | NiFe | — | — | 4 coils | DC, AC uni- or bi-axial pulsed | 140 Oe = 14 mT | 1–20 Hz | 10 min | HeLa4 | Metastases A |
| Disk | Kim et al., 2010 (ref. 22) | 1 μm | 70 nm | Au/NiFe/Au | Anti-human-IL13a2R | — | Electro-magnet | Alternat. | 90 Oe = 9 mT | 10–60 Hz | 10 min | N10 | Glioma A |
| Y. Cheng et al. 2015 (ref. 37) | 2 μm | 70 nm | Au/NiFe/Au | — | — | Halbach cylinder | Rotating | 1 T | 20 Hz | 5–30 min | U87 | Glioblastoma § A | |
| Leulmi et al., 2015 (ref. 38) | 1.3 μm | 80 nm | Au/NiFe/Au | Anti-hCA9 | — | Magnetic stirrer | Rotating | 30 mT | 20 Hz | 45 min | SKRC59 hCA9 | Renal carcinoma A | |
| Muroski et al., 2016 (ref. 39) | 2 μm | 60 nm | SAF8 | — | — | Halbach cylinder | Rotating | 1 T | 20 Hz | 30 min × 3 days | HB1.F3.CD, U87 | Neural stem cell, glioblastoma A | |
| Zamay et al. 2016 (ref. 40) | 500 nm | 60 nm | Au/Ni/Au | AS-9 et AS-14 | — | Coil | Alternat. | 100 Oe = 10 mT | 100 Hz | 10 min | EAC | Elrich cell9 § A | |
| Mansell et al., 2017 (ref. 27) | 2 μm | 118 nm, 70 nm | SAF7 or Au/NiFe/Au | — | — | Halbach cylinder | Rotating | 1 T | 20 Hz | 1 min | U87 | Glioblastoma A | |
| Sphere | Hu and Gao 2010 (ref. 41) | 180 nm + 15 nm | — | Janus nano-composite + Fe3O4 | PS16-b-PAA10 | — | Magnetic stirrer | Rotating | — | 0.83 Hz | 15 min | LNCaP | Prostate tumor A |
| Cho et al., 2012 (ref. 42) | 15 nm | — | Zn–IO | Ab for DR4 | — | 2 magnets | Grad. | 0.2 T | — | 2 h | DLD-1 | Colon cancer § A | |
| Domenech et al., 2013 (ref. 21) | 61 ± 29 nm | — | Fe3O4 | CMDx + EGF | — | Coil | Alternat. | 42 kA m−1 ∼ 52 mT | 233 kHz | 1 h | MDA-MB-23, 184-B5 | Breast cancer, healthy mammary gland A | |
| Wang et al., 2013 (ref. 29) | 0.2–2 μm | Fe | APTES | — | Oscillating magnet | Oscillat. Or Grad. | 160 kA m−1 ∼ 200 mT | 2–10 Hz | 20–60 min | HepG2 | Hepatocellular carcinoma | ||
| D. Cheng et al., 2014 (ref. 32) | 200 nm | — | Fe3O4 | — | — | Electro-magnet | Alternat. | 35 kHz | 0–10–30–60–120 min | HeLa4 | Metastasis A | ||
| E. Zhang et al., 2014 (ref. 43) | 0.1–5.8 μm | — | Fe3O4 | Lamp-1 | — | Coils | Alternat.+ Grad. | 30 mT | 5–20 Hz | 20 min | INS1 | Rat insulinome A | |
| Master et al., 2016 (ref. 44) | 7–8 nm | — | Fe3O4 | PAA(PMA)–PEG ou PAA-P85 | — | Electro-magnet | Alternat. Sinus. | 50 or 100 kA m−1 ∼ 62 or 125 mT | 50 Hz | 30 min or 3 × (10 min ON + 5 min OFF) | MDA-MB-231, BT474, MCF10A | Breast cancer, ductal carcinoma, healthy mammary gland | |
| Wo et al., 2016 (ref. 45) | 250–550 nm | — | Fe3O4 | SiO2/GQD + LB10 | Yes6 | 4 moving magnets | Alternat. | 45 mT | 2000 rpm | 20–60 min | Eca-109 | Esophageal cancer cells | |
| Ju et al., 2016 (ref. 46) | 40 nm | — | Fe3O4 | — | — | Coil | Alternat. | 0.7 mT | 100 Hz | HepG2, Bel-7402, HL-7702 | Hepatocellular carcinoma11, healthy hepatic cell A | ||
| Brossel et al., 2016 (ref. 47) | 100 nm | — | Fe | — | — | 2 magnets | Gradient | 0.66 T | — | 2 h × 21 days | MDA-MB-231 | Breast cancer § | |
| Hapuarachchige et al., 2016 (ref. 48) | 80 nm | — | Fe3O4 | Starch | — | MRI | Alternat. Grad. in bias high field | 9.4 T | 5.4 kHz | 60 min | MDA-MB-231 | Breast cancer | |
| Vegerhof et al., 2016 (ref. 49) | 50–100–200 nm | — | Fe3O4 | PEG + C225 | Yes12 | Electro-magnet | Alternat.+ Grad. | 6.2 G = 0.62 mT | 4 Hz | 15 min | A431 | Skin cancer § | |
| Li et al., 2017 (ref. 50) | 30 nm | — | Fe3O4 | DMSA | — | 2 rotating magnets | Alternat. | 0.1–20 mT | 2–20 Hz | 1 h | MCF-7 | Breast cancer § A | |
| Lunov et al., 2019 (ref. 51) | ∼60 nm | — | Fe3O4 | Carboxy-dextran | Coil | High field pulses of 15 μs | 5.5–8.5 T | ∼1.6 mHz | 100 s | Huh7, Alexander, HepG2 | Hepatocellular carcinoma, liver hepatoma, hepatoblastoma A | ||
| Anisotropic | Chiriac et al., 2018 (ref. 52) | — | 10–200 nm | Fe–Cr–Nb–B | — | — | 4 coils | Rotating or Grad. | 1–20 Oe = 0.1–2 mT | 20–0–70–100 Hz | 5–10–15–20 min | HOS, NHDF | Osteosarcoma, healthy skin cell A |
These materials were chosen by several groups.21,29,32,41,43,44,48,51 However, in order to improve the magnetic actuation efficiency, larger magnetic susceptibilities than those of iron oxides may be required, in particular for low magnetic field operation. Magnetic materials such as nickel,19,33 cobalt,27,39 or NiFe alloys,22,27,36,55 represent good alternatives. To ensure the biocompatibility of particles composed of these toxic metals,56 limited dissolution should be ensured, for instance via a gold coating,57,58 or polyelectrolytes.59
To specifically target a cell type or to increase the particle dispersion in fluids, surface functionalization of particles may be necessary.60,61 Towards this goal, the deposition of a gold layer on the particle surface allows the grafting of organic molecules through self-assembly of thiolates on the gold surface. These thiolates often have a polyethylene glycol (PEG) spacer and functional terminal groups. The PEG spacer increases particle stability, and the functional group allows the anchoring of biomolecules for specific targeting while providing biocompatibility.62 The different surface ligands used in published studies are also presented in Table 1.
But first of all, physical properties of particles play a determining role not only in the effectiveness of the treatment and the particles behavior once they are in place, but also in their ability to target the tumor site, notably in case of intravenous injection. The impact of particles dimension and aspect ratio, when blood flow is used to transport them to the tumor site, is actively investigated. In particular Decuzzi et al., 2010,63 Albanese et al., 2012,64 and Ye, Shen and Li, 2018,65 address the transport mechanisms in blood vessels as a function of particle size and shape; the global review of Wilhelm et al., 2016,66 moreover, analyses the delivery efficiency versus particle physical properties, and the fundamental limitations of particle doses (<1%) that can be delivered to tumors – see below in Section 4.66 Ye, Shen, Yu et al., 2018,28 details the impact of nanoparticles size and shape in their “passive” or “active (subjected to magnetic forces for instance)” transport in the blood stream, in the area of drug delivery. The magneto-mechanical treatment TMMEP, similar in terms of tumor targeting, addresses the same issue of magnetic particles transport in blood flow, for potential clinical applications, as highlighted in the recent review Goiriena-Goikoetxea et al., 2020.24 These studies show that the particle shape – not only the size – clearly plays a key-role in the particle's ability to travel in the blood circulation.24,28,66,67 Non-spherical particles, such as rods or discs, driven in the blood flow, exhibit a propensity to be more efficiently deflected towards the vessel wall, once they have escaped the macrophage uptake. This so-called phenomenon of margination, which consists in the particle lateral drift across the streamlines towards the endothelium, is favoured by an anisotropic shape of the particle, owing to inertial and hydrodynamical forces,68 potentially enhanced by the magnetic actuation.28 Spherical nanoparticles, being much more likely to follow the blood stream lines, however also marginate, as shown in Gentile et al., 2008,69 more efficiently for larger sizes. This expected phenomenon can lead to the particles adhesion on endothelium near the tumour site. The particles are then expected to diffuse from the blood vessel into the tumor tissue through the “leaky” vessel walls, and to be retained in the tumor site, based on the so-called “enhanced permeability and retention (EPR) effect” in tumor vasculature. The anisotropic shape of magnetic particle is again advantageous,70 since generating oscillations due to hydrodynamic or magnetic forces, which leads to a stronger interaction of the particles with the vessel wall, and promotes their transmigration into the tumor.24 The physical properties “size and shape” complemented with the “stiffness and surface functionality” of particles, so-called “4S” parameters,28,65 are therefore decisive for the way particles circulate in the blood stream and penetrate the tumor.28 However, intravenous injection of particles to target tumor sites remains challenging, for any particle shape and size,66 as precised in Section 4.
A compromise is then required between a size that is small enough for in vivo use and large enough to achieve the intended magneto-mechanical effect on the cells. Indeed, the size of particles to be injected intravenously must be small enough not to clog the blood microcapillaries, thus to pass through the pores of blood vessels and to diffuse into tissues.63,71 Moreover, for in vivo uses, particle size is limited by the injection device (to avoid needle clogging). Single SPIONs – fine spherical particles – are particularly appropriate for injections into biological samples, owing to their small sizes less than ∼10–20 nm,72 upper limit for achieving superparamagnetism in Fe3O4 nanoparticles. However, their low magnetic volume limits the magneto-mechanical effects. For a more effective magnetic actuation clustered SPIONs held by a ligand form particles of some 100 nanometers to a few micrometers in sizes.73–75 Besides, the particles in recent studies composed of ferromagnetic layers, in the form of disks or pillars of diameters ranging from a few tens of nanometers to a few micrometers, have boosted studies on TMMEP. Producing larger magnetic forces or torques, such particles have been preferred to SPIONs clusters in various studies involving magnetic actuation on biological cells, despite more complex fabrication techniques.22 The expected efficient actuation, very specific needs of this application in terms of mechanical transfer to the cells, can be fulfilled by these particles, owing to their anisotropic structures. Their potential shape anisotropy and perpendicular interfacial magnetic anisotropy have been used in TMMEP as cited below. Indeed, the magnetization remains quasi-blocked along the anisotropy direction, leading to an efficient mechanical actuation of the particles.
Secondly, combined to the magnetic actuation optimization, size and shape of the particles are chosen to achieve the particles redispersion in zero magnetic field, depending on the material used. Considering the magnetostatic interactions between particles, this property requires particles exhibiting zero or low remanence. Indeed, after their agglomeration in an applied magnetic field, suspended particles can get redispersed when the field is switched off, provided that their magnetic susceptibility remains below a certain threshold, as modelled in our ref. 76 illustrated in our ref. 55. This is particularly the case for SPIONs, which have zero remanence due to their small size yielding superparamagnetic properties, and smaller susceptibility than ferromagnetic particles. Low remanence can also be achieved by controlling the ferromagnetic particle shape and size to obtain, for instance, disk-shaped particles exhibiting magnetic vortices.22,27,77
Finally, we observe for TMMEP various sizes and shapes of particles: from the spherical SPIONs of few nm in diameter,44 the disk-shaped particles of few μm in diameter and few tens of nm in thickness, to the microrods of few μm in length. The sizes and shapes of the particles largely depend on the fabrication techniques, involving either bottom-up approaches (such as chemical routes, usually yielding quasi-spherical particles), or top-down approaches (such as microlithography techniques, usually yielding anisotropic particles, and allowing a wider freedom in the choice of the particles composition, size and shape). Each approach thus leads to specific magnetic and physical characteristics, and to particular experimental conditions in TMMEP, as mentioned here below.
Spheres. Although particles with a shape anisotropy are preferable to transfer a mechanical action on cells, several studies have observed a lethal effect on cancer cells by TMMEP using spherical particles.43,44,46,49–51 It should be noted that such spherical particles may however form small chains due to their magnetostatic interactions in an applied magnetic field, and thus act as a unique elongated particle. The spherical particles used in TMMEP were mainly commercially available SPIONs fabricated by chemical routes, single or in cluster, actuated by applied magnetic fields of low frequency. Additionally, some studies aimed at inducing TMMEP with nanospheres, use either fields of higher frequencies (greater than 5 kHz),21,32,48 or a static field gradient.42,47 More complex structures are also explored to obtain anisotropic architectures, based on nanospheres assemblies. For instance, Hu and Gao developed Janus magnetic nanocomposite-particles, including SPIONs located in a single hemisphere of the particle to create a magnetic shape anisotropy.41
Disks (circles/squares basis). In addition to the spherical SPIONs widely studied in biomedical field for decades, recent disk-shaped particles composed of ferromagnetic layers, have emerged through the techniques of microelectronics, including photolithography and various deposition techniques such as sputtering, evaporation, or electrodeposition, in a top-down approach. They emerged for biological applications in 2008, consisting of synthetic antiferromagnetic (SAF) particles, exhibiting magnetization above those of SPIONs, zero remanence being achieved through the interlayer magnetic coupling, as firstly reported by Hu et al., 2008.78 The SAF structures consist of ferromagnetic multilayers, whose magnetizations are coupled antiparallel through non-magnetic spacer layer, such as ruthenium of thickness chosen in the range 0.6 nm to 0.9 nm. Particles with SAF configuration were firstly assessed as disk-shaped CoFe/Ru multilayers.78 Furthermore, we investigated self-polarization and dispersion phenomena of SAF square-shaped particles, composed of NiFe/Ru multilayers.76 Both SAF multilayers exhibit in-plane magnetization, while more recently, CoFeB/Pt-based SAF multilayers separated by Ru layers were chosen for their out of plane magnetization,79 as outlined below.
In parallel, disk-shaped particles composed of permalloy monolayers (a nickel 80%–iron 20% alloy), exhibiting magnetic vortex configurations, have been investigated and explored to destroy glioma cells.22,80 This innovative study proposed to induce cancer cells apoptosis (see in Section 3, below) through low-frequency vortex-disks vibrations (∼20 Hz).22 Fabricated by the top-down approach, such magnetic disks diameter and thickness have to be optimized to obtain the expected magnetic vortex configuration, as modelled by Guslienko et al.77 This mainly in-plane magnetic structure, provides the expected low remanence and sufficient magnetic susceptibility for an effective magnetic actuation, while remaining below the critical threshold, preventing them from agglomeration.36,55,76,81 This type of vortex-disk particles have been subsequently used in several studies to destroy several types of cancer cells, such as human renal carcinoma cells,38 adenocarcinoma cells and glioblastoma cells.39 They were also used for the first in vivo tests of TMMEP.37
The in-plane magnetized disk-shaped particles (SAF or vortex) may however present a theoretical issue, as highlighted by Mansell et al.,27 since they could tend to orient with their plane in parallel to the plane of rotation of the field, if the surrounding environment permits it. In this hypothetical situation, if their plane is isotropic, magnetization may rotate within the particle plane without transferring mechanical energy to the surrounding environment, leaving the particle motionless. In contrast, when submitted to a rotating magnetic field, particles with out-of-plane magnetization indefinitely rotate with the field. Therefore, they are steadily able to transfer mechanical energy to the medium in which they are embedded.
In a previous study, disk-shaped particles with an out-of-plane SAF configuration were assessed,79 magnetization being perpendicular to the plane of the multilayer interfaces. In good agreement with the hypothesis, a comparative experiment showed that SAF particles with perpendicular magnetization induced a stronger lethal effect on cancer cells than in-plane magnetized permalloy vortices, after the application of a rotating magnetic field for 1 min.27
Nanotubes. Nanowires or nanotubes have an improved shape anisotropy, which strongly tends to maintain their magnetic moment in parallel to the cylinder axis, and thus can yield an efficient magneto-mechanical actuation. The remanent magnetization on this easy axis may be close to 100% of their saturation magnetization Ms,82 depending on the diameter/length ratio. This characteristic, however, leads to a potential aggregation of the particles after the exposure to the magnetic field. The first use of nanowires for a magneto-mechanical effect was tested on breast cancer cells.31 In this study, carbon nanotubes contained 5% of metal impurities that gave the nanowires their magnetic properties. Clustering was observed when particles were subjected to the magnetic field. Moreover, Ni nanowires were used on colorectal carcinoma cells.33 These particles are saturated in a field of less than 250 mT and have, as expected, a very high remanence (about 75% of Ms). Iron and iron oxide nanowires were also tested.32,35 Wong et al.36 studied influence of shape anisotropy, by varying length and diameter of cylindrical particles, from nanodisks to nanotubes shapes. These micromagnetic simulations result in a single vortex structure for particles of diameters ranging from 150 to 350 nm and less than 100 nm in length, in accordance with the characteristics of disk-shaped particles. A double vortex structure appears for a length between 100 and 300 nm, which evolves to a triple vortex structure for lengths greater than 300 nm, a case close to nanowires.36 A strong increase in susceptibility is observed for nanowire-shaped particles (d = 150 nm and l > 400 nm). However, the author36 mentioned the contrast with ferromagnetic vortex discs usually known to show a reduction in susceptibility for a decrease in diameter or an increase in length,77 as likewise experimented in our studies.55,83 Nevertheless, this increase is here associated with an increase of remanence.36 Calculations of the force applied to the cells show an induced force of the order of a few tens of pN for all diameters studied, sufficient to cause apoptosis of the cells (see in Section 3, below).36 In a study comparing rod-shaped particles (d = 50–120 nm and l = 200 nm) with spherical particles (d = 200 nm) of iron oxides, the authors show better efficacy with rods.32 Wang et al.29 also compared rods (d = 80 nm and l = 580 nm) with spherical particles (d = 0.2–2 μm) and also observed a stronger effect with rods. This confirms the key role of the particle-shape anisotropy to transfer the mechanical torque provided by the external rotating or oscillating field to the cells. To allow two types of functionalization on the same particle, nanowires with a gold end were synthesized.34 These particles combining two different surface properties are called Janus particles.
Cubes/spheres chains, and other anisotropic shapes. Although disks, spheres and nanowires are the three most commonly used forms of particles for TMMEP, cubic iron particles doped with zinc were also tested.30 The authors observed an aggregation of chain-shaped particles during the application of the magnetic field, reducing the situation to the case of nanowire-shaped particles. In a similar approach, the nanochains formed by SPIONs subjected to a magnetic field are fixed by depositing a silica shell,75 structures which, unlike nanowires, keep their superparamagnetic properties with a very low remanence. As a new approach, FeCrNbB particles produced by ball milling were used for TMMEP.52 This manufacturing technique consists of grinding a metal powder to obtain anisotropic particles of nanometric size. The obtained size can be reduced between 10 and 200 nm. Besides, they exhibit low remanence and high susceptibility. The main disadvantage is a greater dispersion in size and less accurate control of particle properties. Purity of the initial powder and cleanliness of the manufacturing procedure are important to avoid contamination by other metals.
The systemic delivery also favors potential side effects because of the systemic exposition of the body. For these reasons, and because of the difficulties for a specific molecular targeting, local delivery is becoming the first emerging strategy.
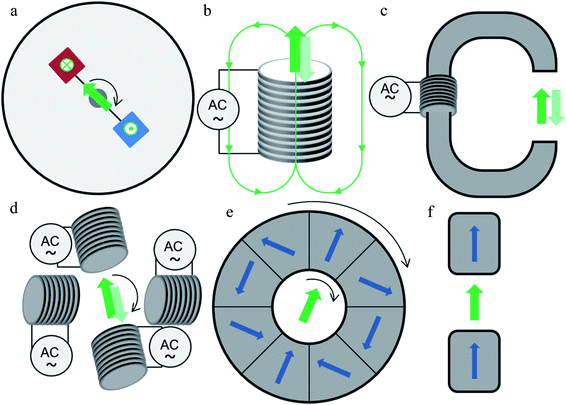
The first method of field application was to use commercial or homemade magnetic stirrers, traditionally used for stirring mixtures with a magnetic bar.19,30,31,38 They are composed of two oppositely magnetized magnets located at each end of a rotating rod with the rotation speed up to 2000 rpm depending on the model (Fig. 3(a)). Advantage of these devices is that they are cheap and already present in most chemistry or biology laboratories. The major disadvantage is the inhomogeneity of the applied magnetic field. Indeed, a field mapping carried out on a commercial magnetic stirrer shows that right above the magnets, the field is perpendicular to the plane of the stirrer and has a value of 30 mT.84 Although the term “rotating magnetic field” is generally used to refer to this type of device, the field acting on the particles located above the circular trajectory of the rotating magnets is actually pulsed up and down during the rotation of the magnets. Moving away from the trajectory by 1 cm outwards, the field strength decreases and an in-plane component appears, of about 5 mT. In the center of the agitator, the field is parallel to the plane and has a value of 15 mT, which is indeed a rotating field. In the rest of the literature, the field strength values applied with magnetic stirrer are given between 30 and 240 mT but the location of the measurement (center or above the trajectory of the magnets) is not indicated.19,31,38,41,85 This device can only be used in vitro, taking care to distribute the cells to be treated over the path of the magnets for a pulsed vertical field, or in the center of the agitator for a rotating horizontal field. In both cases, the inhomogeneity of the field and the induced field gradient must be considered. Similarly, a system also based on the rotation of two magnets is used by Li et al.50 In this case, the sample was placed between the ends of two rotating rods. The two rods were aligned and a magnet was placed at the end of each rod in opposite directions. The applied field was adjusted by varying the distance between the two rods with a maximum of 50 mT. Wo et al.45 developed a more complex system using four permanent magnets placed on a disk under the culture plate producing a 45 mT field on the cells. During magnetic field exposure, the disk rotates and can also move on axial and radial direction.45 Moreover, Maniotis et al.26 recently developed a versatile system for TMMEP, configured with two to eight permanent magnets inserted in a rotating turntable, leading to field amplitudes of 200 mT and mean field gradient of 45 T m−1.
A method widely used to apply an alternating magnetic field to cells consists in using coils or electromagnets (Fig. 3(b)–(d)). The field can be applied with an iron stick placed between the wells of a culture plate, itself wound with copper wire, to apply a 90 Oe (=9 mT) field.22 Alternatively, the culture plate can be directly placed above a coil (Fig. 3(b)).33,46,49,51 Kilinc et al.34 used a Fe–Co–V tip wound with a copper coil to apply the magnetic field (the amplitude is not indicated).34 In the latter case, the magnetic field was applied very locally (500 μm from the tip). Magnetic fields produced by these three methods are highly inhomogeneous and decrease sharply as a function of the distance from the field source. Another method consists in placing the sample in the air gap of a U or C-shaped ferrite core subjected to alternating current (Fig. 3(c)). This technique was chosen by D. Cheng et al.32 and used at very high frequency (35 kHz) on cells detached from the support and placed in a tube; and by Martínez-Banderas et al.35 to apply a 1 mT field at 10 Hz. With this method, the magnetic field is homogenous in the air gap but limitations come from the size of the device compared to the field amplitude. In a different approach, cells or mouse are placed directly in the center of the solenoid to apply a 100 Oe (=10 mT) field.40 Here again, the field amplitude is very limited. Several commercial devices composed of an induction system with a ferromagnetic core or several coils have also been used.21,43,44 The field produced by these three systems creates a gradient that is either used by some authors or avoided by positioning the cells with respect to the field source geometry. The use of coils of identical size and even number, placed around the area of interest, creates a fairly homogeneous field around the symmetry plane separating them. This is referred to as Helmholtz coils (Fig. 3(d)). This configuration was used to apply a 140 Oe (=14 mT) uni- or bi-axial pulsed field.36 The disadvantage of this method is the rapid heating of the coils when a high current is applied, which requires a cooling system.
In order to produce a homogeneous field of larger amplitude, a Halbach cylinder can be used (Fig. 3(e)). This cylinder is composed of several permanent magnets (usually 8, 12 or 16) suitably oriented to produce a uniform magnetic field in the hollow of the cylinder. Using a rotation system, this cylinder was used to apply a rotating field in its center of about 1 T on cells, but also on mice.25,27,37,39 This device allows stronger fields to be applied in a limited space. The field rotation frequency is determined by the cylinder rotating speed which can be adjusted as required and is only limited by mechanical constrains (motor, generator, mechanical and magnetic forces, magnets weight).
In an innovative approach, a preclinical MRI system was used to apply a pulsed gradient.48 The main advantage of this method is its compatibility for subsequent clinical use, as MRI imaging systems are already widely used in hospitals. The field strength applied here is 9.4 T at a frequency of 5.4 kHz.
As mentioned above, while most of the studies discussed here focus on an oscillating or rotational motion of particles, some studies aimed solely at creating static forces pulling on the cell-membrane-bound particles, through the application of a static magnetic field gradient.42,47 In this case, static magnetic field gradients are applied using two permanent magnets placed on either side of cells or mouse (Fig. 3(f)). The maximum field created is between 0.2 and 0.66 T. However, in a study comparing the effects of an oscillating field and a field gradient, the oscillating field showed a better efficacy.29 The magnetic field of amplitude 160 kA m−1 (i.e.B ∼ 200 mT) was here applied by a magnet moved alternatively away or closer to the sample to produce the oscillating field and field gradient.
3. Effects on cancer cells in vitro
The various methods described above aim at reducing the viability of cancer cells, focusing on various cancer types and cell lines as presented in Table 1; their efficacy was assessed via counting the number of viable cancer cells, through different techniques as described below (in Section 3.1). The main percentages of cancer cells viability, extracted from the reported studies, have been summarized in Table 2. Some experimental parameters, in particular the conditions of magnetic field application, have been analyzed below in Section 3.1, correlated with the reported viability results.In addition, a number of these studies describe the biological mechanisms at the cell level that could explain the observed decrease in cancer cell viability. Depending on the targeted internal/external part of the cell, the mechanical stress may locally damage the cell membrane (compromising its integrity, modifying its permeability), or may cause internal disturbances in the cytoplasm, including in particular perturbations of the lysosomes or cytoskeleton, as noted in Table 2 column 6, and described below in Section 3.2.
| References | Experiment conditions | Test 1 | Test 2 | Test 3 | Main effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Particles + field | Particles | Control | Particles + field | Particles | Control | Particles + field | Parti-cles | Control | ||||||
| a Abbreviations: TB: trypan blue; CCK8 = WST-8 assay; Resaz.: resazurin assay, Lucif.: luciferase assay. NP: nanoparticle. EGF: epidermal growth factor. b Mainly mentioned effects of TMMEP on cells: CM: cellular membrane perturbation, (through membrane-bound or internalized particles); LP: lysosome perturbation (including on lysosomal membrane); CK: cytoskeleton and cytoplasm perturbation; CL: cell lysis; IC: intracellular perturbation. c Potentially initiating cell death pathways, in particular: /A: apoptosis; /Nr: necrosis or membrane rupture (leakage); /—: cell death, undefined pathway; CD: cell detachment. | ||||||||||||||
| Fung et al., 2008 (ref. 19) | MTT | 11% | 100% | CK /— | ||||||||||
| Kim et al., 2010 (ref. 22) | LDH | ∼10% | ∼90% | CM /A | ||||||||||
| Hu and Gao, 2010 (ref. 41) | TB | 23% | 99% | 100% | CM /A | |||||||||
| Liu et al., 2012 (ref. 31) | 75 mT | TB † | 70% | 92% | 100% | PI | ∼80% | ∼98% | LDH | ×1.61 | ×0.9 | ×1 | CM & CL /— | |
| 40 mT | TB † | 83% | 92% | 100% | PI | ∼83% | ∼98% | LDH | ×1.87 | ×0.9 | ×1 | |||
| Cho et al., 2012 (ref. 42) | CCK8 | ∼48% | ∼95% | CM /A | ||||||||||
| Wang et al., 2013 (ref. 29) | Nanowire | TB | 65–70% | ∼95% | CM /Nr | |||||||||
| Sphere | TB | ∼70% | >95% | |||||||||||
| Domenech et al., 2013 (ref. 21) | NP + EGF | Resaz. | ∼70% | 100% |
|
LP /A | ||||||||
| NP | Resaz. | ∼120% | 100% | |||||||||||
| NP + EGF | Resaz. | ∼100% | 100% |
|
||||||||||
| NP | Resaz. | ∼100% | 100% | |||||||||||
| E. Zhang et al., 2014 (ref. 43) | NP function. | 7-AAD | 98% | 100% | LP /A | |||||||||
| NP | 7-AAD | 99% | 100% | |||||||||||
| D. Cheng et al., 2014 (ref. 32) | Rod 1 h | TB | ∼76% | ∼93% | MTT | ∼70% | 100% | CM /A | ||||||
| Rod 2 h | TB | ∼60% | MTT | ∼70% | ||||||||||
| Sphere 1 h | TB | ∼89% | ∼95% | MTT | ∼92% | 100% | ||||||||
| Sphere 2 h | TB | ∼85% | MTT | ∼88% | ||||||||||
| Contreras et al., 2015 (ref. 33) | 1 kHz, 30 min | MTT | ∼62% | 100% | LDH | ∼66% | ∼88% | CM /Nr | ||||||
| 1 Hz, 30 min | MTT | ∼67% | LDH | ∼66% | ∼88% | |||||||||
| Master et al., 2016 (ref. 44) | Breast cancer | MTT | ∼50% | ∼95% | CK /— | |||||||||
| Breast cancer | MTT | ∼25% | ∼85–95% | |||||||||||
| Healthy cells | MTT | ∼82% | ∼95% | |||||||||||
| Y. Cheng et al., 2015 (ref. 37) | MTT | 40% | 80% | 100% | 7-AAD | ∼10% | CM /A | |||||||
| Kilinc et al., 2015 (ref. 34) | 0.5 Hz | Optical | ∼50% | ∼97% | CM /— | |||||||||
| Leulmi et al., 2015 (ref. 38) | TB † | ∼20% | — | 100% | CM /A | |||||||||
| Wo et al., 2016 (ref. 45) | 20 min | Cell | ∼74% | 100–105% | CM /— | |||||||||
| 60 min | Titer | ∼58% | 100–105% | |||||||||||
| Muroski et al., 2016 (ref. 39) | NSC | MTT | 25% | 58% | CM /A, /CD | |||||||||
| Glioblastoma | Lucif. | ∼37% | 100% | |||||||||||
| Martínez-Banderas et al., 2016 (ref. 35) | NP + doxo. | Resaz. | ∼27% | ∼37% | ∼100% | IC /— | ||||||||
| NP | Resaz. | ∼65% | ∼95% | ∼100% | ||||||||||
| Hapuarachchige et al., 2016 (ref. 48) | MTS | ∼73% | ∼95–97% | IC /—, CD | ||||||||||
| Vegerhof et al., 2016 (ref. 49) | TB | ∼49% | — | 100% | CM /Nr | |||||||||
| Wong et al., 2017 (ref. 36) | 1–5 Hz | TB † | ∼50% | — | ∼87% | Resaz. | ∼73% | 100% | BrEth | ∼60% | ∼92% | CM /A | ||
| Li et al. 2017 (ref. 50) | 20 Hz 20 mT | MTT | ∼75% | >95% | CM /A | |||||||||
| Mansell et al., 2017 (ref. 27) | Vortex, 1 min | TB | ∼88% | ∼99% | CM, IC /A, Nr | |||||||||
| SAF, 1 min | TB | ∼38% | ∼99% | |||||||||||
| Shen et al., 2017 (ref. 30) | Day 1 | CCK8 | ∼65% | ∼130% | PI | ∼43% | ∼98% | ∼98% | CM, LP /A, Nr | |||||
| Day 2 | CCK8 | ∼40% | ∼80% | |||||||||||
| Day 3 | CCK8 | ∼10% | ∼50% | |||||||||||
| Chen et al., 2020 (ref. 25) | TB | ∼30% | ∼80% | ∼100% | CM, LP, IC /A | |||||||||
| Zamay et al., 2016 (ref. 40) | With AS-9 and AS-14 | TB | ∼10% | ∼70% |
|
CM /A | ||||||||
| Without | TB | ∼35% | ∼70% | |||||||||||
| With AS-9 and AS-14 | TB | ∼70% | ∼80% |
|
||||||||||
| Without | TB | ∼60% | ∼80% | |||||||||||
| Chiriac et al., 2018 (ref. 52) | 20 Hz, 20 min | MTT | ∼55% | ∼95% | CM, LP, IC /A | |||||||||
| Lunov et al., 2019 (ref. 51) | 8 T 15 μs pulses | WST-1 | ∼40% | ∼105% | ∼95% | LP /A | ||||||||
| Ju et al., 2016 (ref. 46) | Drug delivery + NPs 100 Hz | — | CM /A | |||||||||||
In most cases, these perturbations on cells are correlated with the expected and experimentally observed cell death pathways, induced by TMMEP (see Section 3.3 and Table 2). Among the different forms of cell death, largely investigated over the last two decades,86–88 apoptosis and necrosis cover a wide range of cellular processes, with however a full range of features from fully necrotic to fully apoptotic.86 Apoptosis, – referred as “programmed cell death” or “cellular suicide” – potentially purely physiological in the absence of external perturbation, can also be triggered in response to external perturbations in the extra- or intracellular microenvironment.86 It is worth remembering that the elimination of apoptotic cells, engulfed by phagocytes, generally takes place without any inflammatory response in tissues, while necrosis is well known to induce inflammatory reactions.87,89 This regulated cell death represents one of the essential natural process maintaining homeostasis of healthy tissue or organisms.86–88 By contrast, the apoptosis pathway is well known to be often defective in cancer cells, for which evading apoptosis is a factor promoting their proliferation.90,91 Precisely here, most of the reported works show that TMMEP can trigger cancer cell death through apoptosis pathway, some of them through necrosis, as shown in Table 2 (column 6), and Section 3.3.
All these studies were conducted on two-dimensional in vitro cell models, except Lunov et al.,51 who utilized 3D multicellular aggregates to assess TMMEP efficacy.
3.1 Cellular viability
- Assays based on the staining of dead cells: trypan blue (TB) is a cell permeant dye, which is released out of live cells. Live (non-colored) and dead (blue-colored) cells are then counted, manually under either a microscope, or automatically using a haemocytometer. This assay is distinguished from those where the dye enters only in dead cells: propidium iodide (IP), ethidium bromide (BrEth) and 7-AAD (7-aminoactinomycin D), which stain nuclei of cells that have lost their membrane integrity. These dyes are DNA/RNA intercalating agents, which become fluorescent upon intercalating. After incubation with the dye, the cell fluorescence is measured using flow cytometry.
- Assays evaluating the metabolic activity of cells: MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium), CCK8 (Cell Counting Kit) meaning WST-8 assay, WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) and MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium), are tetrazolium salts that are reduced by cellular NADH (nicotinamide adenine dinucleotide (NAD) in reduced form), forming formazan compounds that absorb light at specific wavelengths. The resazurin (Resaz.) assay (present for example in CellTiter-Blue® or PrestoBlue®) is reduced in metabolically-active cells into resorufin, which is pink and fluorescent.
- Assays based on the leakage of an enzyme from cells having impaired plasma membrane integrity, such as the LDH assay, in which the release of cytoplasmic LDH out of the cells is quantified after reacting with a tetrazolium salt.
- Assays combining two of these dyes, such as the LIVE/DEAD® test (L/D). It is based on the use of acetoxymethyl calcein (AM) which stains live cells and ethidium homodimer-1 (EthD-1) which stains dead cells. In metabolically-active cells, non-fluorescent calcein AM is converted by intracellular esterase activity to green fluorescent calcein. EthD-1 enters cells with impaired membranes and binds to nucleic acid, leading to red fluorescence.
In some studies, the number of live cells is quantified using optical microscopy and compared to the number of cells in untreated control. Some markers of the cell nuclei are also used, allowing to differentiate healthy cells from affected ones, which are characterized by dense nuclear staining and atypical shape.34 Finally, the light intensity emitted by cells expressing luciferase is used in one study. This enzyme catalyzes bioluminescence reaction, it is used to quantify the number of cells.39
Some nanoparticles show interference with most of these assays. For instance, the presence of particles in cell cytoplasms impairs the appropriate counting of blue cells in the trypan blue assay when automatic counting is used. To use trypan blue for such application, cells should be manually counted under a microscope, taking care to differentiate between blue colored cells and particle charged cells that appear darker under the microscope. It has been shown that MTT, MTS, LDH and resazurin tests can interfere with some particles.92,93 and that absorbance and fluorescence measurements can be affected by nanoparticles showing intrinsic absorbance or fluorescence property.93 Therefore, when working with particles, the cell viability assay has to be carefully chosen so that interference is minimal. It is recommended that several different toxicity assays should be implemented.
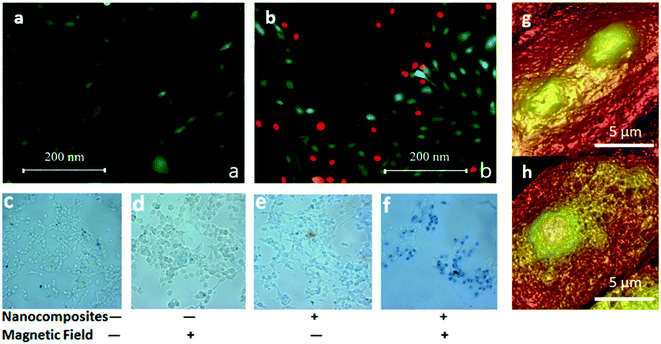 | ||
| Fig. 4 (a) and (b) Extracted from Chiriac et al., 2018:52 human osteosarcoma cells (a) before and (b) after the magneto-mechanical actuation (rotating field). Live cells are colored in green and dead cells in red [Reproducted with permission (ref. 52), Copyright© 2018, Springer Nature, Sci. Rep.]; (c–f) extracted from Hu and Gao, 2010:41 prostate cancer cells after treatment: (c) cells only, (d) cells exposed to magnetic field, (e) cells with particles, (f) cells with particles and exposed to magnetic field. Particles are biphasic iron oxide nanocomposites (d = 180 nm). Rotating magnetic field (0.83 Hz) was applied for 15 min. Dead cells appear blue due to trypan blue staining [Reproducted with permission (ref. 41), Copyright© 2010, American Chemical Society, J. Am. Chem. Soc.]; (g and h) extracted from Liu et al., 2012:31 cell membrane topographical imaging by AFM. (g) Control group. Surface of untreated cell was smooth. (h) MCF-7 cell treated by multiwalled carbon nanotubes exposed in 40 mT magnetic field for 20 min. Surface of the treated group is much rougher than controls with many small pore like structures [Reproducted with permission (ref. 31), Copyright© 2012, American Chemical Society, Nano Lett.]. | ||
Results presented below show the lowest value of cell viability obtained, for each publication, when several conditions or parameters have been tested (Table 2).
Table 2 shows the wide variety of results obtained in the different published studies. Although not quantified, a significant decrease in cell viability after TMMEP was also observed with a 7-AAD test,39 and a LIVE/DEAD test.48
In summary, no clear correlation between the observed effect and the used parameter can be derived from the comparison of these different studies. However, initial conclusions can be proposed, from some parameters tested, as described thereafter.
Influence of the field frequency on cell death. Most studies were carried out at low frequencies, between 10 and 50 Hz. A few studies have also tested very low frequencies between 0.5 and 5 Hz (see Table 1). These low frequencies are in contrast to the very high frequencies (above 1 kHz) used in some studies, which correspond to the frequencies commonly used for magnetic hyperthermia. These studies are cited here because the authors were interested in the induced mechanical effects and do not show any temperature increase during magnetic field application. In some work, the influence of magnetic field frequency on cell death induction was studied while keeping all other parameters constant. In the first study, conducted in 2010, the authors Kim et al.22 showed that the optimal effect was obtained with frequencies of 10 and 20 Hz, inducing ∼90% of cell mortality. The effect obtained was lower at 40 Hz (∼75% of cell mortality), significantly decreased at 50 Hz (∼25% of cell mortality) and non-existent at 60 Hz.22 The effect of lower frequencies (from 1 to 20 Hz) was assessed by Wong et al.36 in 2017, which showed a slight increase in lethal effect as the frequency decreases,36 with ∼80% of viable cells at 10 Hz and ∼73% of viable cells at 1 Hz. However, these results contrast with those of Li et al.,50 published in the same year, showing a better efficiency at 20 Hz, with 75% of cell viability, than at 2 Hz, with 80% of cell viability.50 Similarly,Wang et al.29 showed a better effect at 10 Hz compared to 2 and 5 Hz. Frequencies above 20 Hz were studied by Chiriac et al.,52 in 2018. They showed a higher efficiency for a 50 Hz frequency with ∼68% cell viability, compared to frequencies of 20, 70 and 100 Hz which gave cell viability of at least 75%.52 In a study on colorectal cancer cells, a very low frequency (1 Hz) was compared to a much higher frequency (1 kHz), but with an applied magnetic field of only 0.5 mT while the particles saturation field is 250 mT.33 The authors concluded that the efficiency is slightly better with a 1 kHz treatment with ∼62% cell viability compared to ∼67% of cell viability with a 1 Hz treatment, although this difference is not statistically significant. The similarity of the effect obtained with these two very different frequencies raises the question of the mechanisms involved. Indeed, it is possible that particles subjected to each frequency may be effective in causing a lethal effect, but through very different mechanisms.
Influence of the duration and repetition of magnetic field exposure. The magnetic field application duration varies from 1 min to 2 h and is sometimes repeated for several days (see Table 1). In a study published in 2015, a magnetic field of 20 Hz was applied in vitro for 5 or 30 min.37 The authors showed an almost identical effect on cell viability after treatment (10.7% and 13% viability, respectively) with these two durations. In their in vivo study, the field was applied for 1 h and repeated daily during 1 week. In another in vitro study, a field of higher-frequency (35 kHz) and duration of treatment ranging from 10 to 120 min were used; the authors showed a greater decrease in cell viability with increasing exposure times, reaching 30% of decrease for 1 and 2 hours of treatment.32 Similarly, a comparison between 10 and 30 min of treatment showed a better in vitro effect for 30 min,33 although the best effect obtained was a 5% decrease in cell viability, which is rather low. Exposure times of less than 20 min were also tested,49,52 and revealed a linear relationship between cell viability and exposure time. In conclusion, cell viability decreases with increased time of exposure to magnetic field. This effect seems to saturate beyond 1 hour of treatment.
By repeating the treatment daily (20 Hz field for 20 min), Zhang et al.43 showed decrease of cell number compared to controls. However, it would be interesting to compare this result, obtained after repeated exposure, to the result that would be obtained after a single treatment. Similarly, Muroski et al.39 showed a decrease in cell viability after three exposures to the magnetic field (20 Hz field for 30 min), but not compared to a single exposure.
A “pulsed” mode was also tested.44 The magnetic field was applied for 10 min and then stopped for 5 min, with a total exposure time of 30 min. A higher decrease in cell viability was observed compared to continuous field application. Tested on two cell lines, cell viability decreased to ∼25 and ∼50% with pulsed mode versus ∼50 and ∼100% with continuous mode, for a particles concentration of 0.05 g L−1.44
3.2 Targeted cancer cell components and cell activity affected by the treatment
3.3 Main cell death pathways and processes observed in TMMEP
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAX proteins that decreases after treatment, indicating a greater amount of pro-apoptotic proteins. Although not quantified, the presence of apoptotic cells was also confirmed by increased intensity of Hoechst 33258 staining of cell nuclei, probing chromatin condensation.50
BAX proteins that decreases after treatment, indicating a greater amount of pro-apoptotic proteins. Although not quantified, the presence of apoptotic cells was also confirmed by increased intensity of Hoechst 33258 staining of cell nuclei, probing chromatin condensation.50
3.4 Comments on the in vitro apoptotic occurrence in TMMEP
Clearly, whereas all treatments aim at minimizing the cell viability, the in vitro outcomes exhibit the great variability from one study to another (see Table 2). The diversity of the cellular responses can easily be understood, given the diversity of experimental conditions (Tables 1 and 2), as commented above.Although without statistical validation, we can observe some trends based on all the in vitro studies. As shown in Table 2 (column 6), apoptosis represents, in most cases, the latest stage of the above cited cellular perturbations,21,22,25,27,30,32,36–43,46,50–52 however with variable rates of cancer cells mortality (or viability). Some of the studies show the induction of necrosis only – such as necrotic membrane disruption, cell membrane leakage or cell lysis.29,33,49 Other studies mention both apoptosis and necrosis as cell death pathway.27,30 The cell death pathway may likewise remain unspecified, neither apoptotic nor necrotic,19,31,34,35,44,45,48 opening the door to further studies.19
Concerning the influence of particle shape, studies using disc-shaped particle have all highlighted apoptotic pathways for the treated cancer cells (see in Table 1, noted “A” in the last column, and the viability percentage in Table 2). Several of the studies using spherical particles likewise observe apoptosis pathways, however not all of them. Nanorods have a greater propensity to induce necrosis, or more generally to induce more disruptive death pathway than apoptosis, such as membrane lysis.
Most published studies investigated classical molecular pathway as previously done for chemotherapy and targeted therapies. However, several groups recently described the existence of specific molecular pathway responding to mechanical forces. This open a new field of exploration that will be mandatory in the magneto-mechanical field – Broders-Bondon et al., 2018.98
4. In vivo studies
Several groups studying TMMEP performed in vivo tests, a necessary step to validate the efficacy of this treatment. These studies are very important, when we integrate the major discrepancies observed between in vitro and in vivo efficacy in classical cancer therapies. This discrepancy might be much more important here, because of the major physical differences between liquid in vitro and the in vivo tissue micro-environment.The tumor targeting, in living organisms, may be based either on a systemic injection (intravenous), or on a local injection of the particles within the tumor site. As indicated above in Section 2.2, anisotropic shapes of particles are much more suitable than nanospheres for their transport through the bloodstream.24 However, considering potential clinical applications, intravenous administration of particles to target the tumor site remains challenging. The in-depth analysis of Wilhelm et al., 2016,66 shows the weak proportion of injected particles in the blood flow which penetrate the targeted tumor – less than 1%, so far – regardless of the particle shape and size. Biological mechanisms leading to the particles engulfment by phagocytic cells, which occur mainly in organs such as the liver, spleen and lungs,63,66 highly contribute to eliminate particles from the blood circulation, preventing them to be delivered to the tumor site.24,66 According to their size, particles may also be eliminated by the kidneys, lymph nodes and skin. Because of this systematic loss of particles, approaches utilizing a systemic administration of particles still require to be improved for clinical applications. The few TMMEP in vivo studies available in literature, presented in Table 3, show different modes of particles administration. The local injection of particles predominates – with two cases of particles previously mixed with the cancer cells for a common injection – leading to upstream level studies. One of them uses the intravenous mode, assuming that the tumor targeting and particles accumulation within the tumor site was helped by the EPR effect.49 However, we can also consider that a direct injection of the magnetic particles into the tumor site is interesting for potential clinical applications. The TMMEP may then be the way to avoid the surgical removal of small tumors, or to act repeatedly after surgery on the remaining or recurrent cancer cells targeted by the particles.
| Ref. | Model | Particle injection | Molecule | Magnetic field | Analysis | |||
|---|---|---|---|---|---|---|---|---|
| Timing | Procedure | Beginning | Duration | Timing | Procedure | |||
| Cho et al., 2012 (ref. 42) | Zebrafish | Day 0 | 2.5 ng injected in the vitellus | 24 h after NP | 24 h | Immediately after MFE | Tail angle, apoptosis | |
| Y. Cheng et al., 2015 (ref. 37) | Athymic nude mice 1 × 105 U87 cells in orthotopic model (brain) | Day 0 | 50 NP/cell injected with cells | From day 4 | 1 h × 7 days | Along study | Survival study, tumor volume monitoring via luciferase | |
| Day 3 | 5 × 106 NP injected intra-tumoraly | From day 4 | 1 h × 7 days | Immediately after MFE | Apoptosis observation, HE label | |||
| Zamay et al., 2016 (ref. 40) | ICR white mouse 1 × 106 EAC cells in the leg | Day 7 | 2 × 107 NP/100 μl PBS injected intra-tumoraly | AS-9 and AS-14 | 1 h after NP | 10 min | 4 h after MFE | HE label |
| Vegerhof et al., 2016 (ref. 49) | Nude mice 2 × 106 A431 cells subcutaneous | D > 4–5 mm | 6 mg of NP in 200 μl injected intravenously | Cetuximab | 2 h after NP | 30 min × 7 in 14 days | Week 3 | Tumor volume monitoring |
| Brossel et al., 2016 (ref. 47) | BALB/C mice 1 × 107 MDA-MB-231 cells subcutaneous | Day 0 | 5 mg of iron injected with cells | V > 0.1–0.2 cm3 | 2 h × 21 days | Different timing | Tumor volume monitoring, HE label | |
| Li et al., 2017 (ref. 50) | C75BL/6 mice 1 × 105 MCF-7 cells subcutaneous | D > 5 mm | 2 mg of NP (in average) injected subcutaneously | 8 h after NP | 1 h | 24 h after MFE | HE label | |
| M. Chen et al., 2020 (ref. 25) | Athymic nude mice 5 × 106 U87 cells subcutaneous | Day 0, 2 and 4 | 5 mg kg−1 of NP injected intra-tumoraly | From day 1 | 30 min × 6 in 14 days | Day 21 | Tumor volume monitoring, apoptosis observation, HE label | |
| Athymic nude mice 2 × 106 U87 cells in orthotopic model (brain) | Day 0 | 2.5 mg kg−1 of NP injected intra-tumoraly | From day 2 | 20 min × 5 days | Along study | Survival study, HE label | ||
The in vivo studies are summarized in Table 3. Details on particle material and field application were reviewed in Table 1.
In 2012, Cho et al.42 studied the effect of applying a constant magnetic field of 0.5 T creating a gradient, for 24 h, on spherical zinc-doped iron oxide nanoparticles injected into zebrafish. Particles were functionalized to target the DR4 receptor (Death Receptor 4, which may be involved in triggering apoptosis) and were injected into the yolk at the embryonic stage. After magnetic field application, morphological alterations were observed in the tail, which had developed at an angle of 22°. Caspase 3 was studied here as a marker of apoptosis and a 6-fold increase in the number of caspase 3 positive cells was observed in zebrafish exposed to the magnetic field.42
The first in vivo rodent study was conducted by Y. Cheng et al.37 on a mouse model of glioblastoma by orthotopic grafting (cancer cells were injected into their original organ, here the brain). The mice survival assay used magnetic particles incubated with the glioma cells prior to the tumor implantation. By applying a rotating field of 1 T at 20 Hz for 1 h daily for 1 week, the authors showed an increase in median survival from 56 days for the group exposed to particles without magnetic field application, to 63 days for the treated group with magnetic field, as shown in Fig. 5. Median survival for a group of control mice that would have been exposed neither to particles nor to the magnetic field is replaced here by mice submitted to particles only without field, the non toxicity and non efficiency of particles alone being verified. In this preliminary approach, vortex particles were injected at the same time as tumor cells and the field was applied from the 4th day post-implantation. Although anti-cancer treatments are usually tested from the 10th day, tumor size significantly increasing from this time.99,100 Here, the authors37 showed a significant decrease in tumor volume for 60% of mice on the day 28, using the fluorescence intensity of luciferase. To understand the involved mechanisms, a histological study was carried out on brain sections after a daily treatment of 30 min for 1 week, the particles being injected directly into the tumor on the 3rd day, i.e. 24 h before the beginning of exposure to the magnetic field. The authors37 likewise showed a 19% increase in the number of cells in apoptosis. Almost all the particles are still located in the tumor 7 days after injection, and no particles have been found in other healthy organs (kidney, liver, lungs, large intestine, heart, bladder, spleen, testicles).
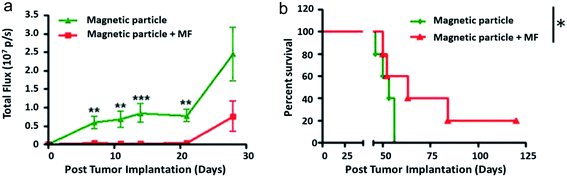 | ||
| Fig. 5 Extracted from Y. Cheng et al., 2015:37in vivo therapeutic efficacy of the magnetic particles (MPs) under rotating magnetic field. “The U87 cells were pre-incubated with MPs for 24 h and implanted in the mouse brain”. (a) Quantification of the tumor bioluminescence signal over 4 weeks (n = 5 mice per group). Data are presented as mean ± SE. **p < 0.01, ***p < 0.001 (Student's t test); (b) Kaplan–Meier survival curve of the mice with and without magnetic field treatment. *p < 0.05 (log rank test) [Reproducted with permission (ref. 37), Copyright© 2015, Elsevier B.V., J. Control. Release]. | ||
In a subcutaneous tumor model, Vegerhof et al.49 showed significantly reduced tumor growth after application of a non-uniform field of 6.2 G (i.e. 0.62 mT) at 4 Hz for 30 min, repeated for 7 days.49 The amplitude of the field is very small as compared to other published studies. Spherical magnetite particles of different sizes were injected intravenously about 2 hours before field application. Particles with a diameter of 200 nm functionalized with an antibody (cetuximab) to EGF receptors were most effective with a tumor growth of only 32% after 6 days of treatment compared to a growth of 548% for the particle-free control group with cetuximab injection alone. The hysteresis loops of particles could help in the understanding of the results.
Also in a subcutaneous model, Li et al.50 observed morphological changes after the application of a magnetic field from 1 to 10 mT with a frequency varying between 2 and 20 Hz for 1 h. Spherical iron oxide particles were injected into the subcutaneous tumor and was exposed to the magnetic field 8 hours after injection. Euthanasia was performed 24 hours later and tissues were analyzed by a Hematoxylin–Eosin (HE) label that allows the observation of cell morphology. The authors showed that tissues of control groups (saline injection and field exposure or particle injection alone) were normal while tissues subjected to particle injection and magnetic field appear destroyed. The effect was maximal at the highest frequency (20 Hz) as well as the highest field strength (20 mT).50
In vivo tests were also performed by Zamay et al.40 by injecting adenocarcinoma cells into the mouse thigh. One hour after intra-tumor injection of the particles (nickel nanodiscs), mice were subjected to a field of 100 Oe (i.e. 10 mT) at 100 Hz for 10 min. Samples were collected 4 hours after the field application. Analysis of histological sections showed that injection of AS-9 and AS-14 aptamers lead to cancer cells destruction but that this effect was increased when the magnetic field is applied.40 Monitoring tumor size by observing tumor pictures during treatment (injection of particles and/or aptamers + field application) repeated 3 times in 3 days showed that aptamers alone do not reduce the tumor volume. Application of magnetic field on non-functionalized particles caused destruction of the tumor but also of the muscles and epithelium, causing tissue necrosis. These phenomena are also visible after the field is applied to functionalized particles but appear from the 3rd day of treatment on the example shown here. In this type of assays, tumor and tissues imaging remains challenging.
In a different approach, the application of a field gradient was studied by Brossel et al., 2016.47 Iron nanoparticles were injected with cancer cells subcutaneously. A field gradient was applied from day 18 for 2 hours and repeated for 21 days. Authors showed a significant reduction in tumor volume compared to controls (median volume of 529 mm3 for treated group versus 1334 mm3 for control groups).
5. Discussion – summary
Although many advances have been made in the area of cancer therapy, the severe side effects of numerous treatments have oriented the research towards the development of targeted and local treatments. In particular, magnetic nanoparticles, already involved for decades in hyperthermia studies, recently show their strong potential to destroy cancer cells through their mechanical vibrations, remotely actuated by an alternating magnetic field at low frequency (a few tens of Hz). This review have identified and compared the studies, mostly in vitro, and the very first in vivo ones, focusing on this recent magneto-mechanical approach. Magnetic particles transfer very locally the energy of their mechanical vibration induced by the applied magnetic field, to the neighboring biological entities of micro- or nanometric dimensions. Studies reported here show the particles potential of destruction on different types of cancer cells in culture and in tumors (tested on a variety of cancer types such as glioblastoma, breast cancer, fibroblast, renal/hepatocellular/colorectal carcinoma, prostate tumor, insulinome, esophageal/skin cancer, osteosarcoma, etc.). The aim and ability of targeting malignant cells while sparing healthy ones is emphasized.In most of the in vivo studies, magnetic particles are injected directly into the area of interest and therefore do not pass through the bloodstream. As long as the functionalization strategies for reaching the zone of interest are not sufficiently effective, it remains difficult to plan to inject the particles by venous route for potential clinical applications. Although venous injection remains a challenge for targeting the tumor, the size and especially the shape chosen for the particles – with the help of appropriate functionalization – could be determinant for their circulation in the blood flow, at least near the tumor site. Anisotropic shapes – nanodiscs or nanorods, nanowires – should be more favorable than the spherical ones, owing to the phenomenon of margination in the flow. Moreover, anisotropic particles penetrate more efficiently into the tumor site, owing to the EPR effect, increased if magnetically actuated by an alternative magnetic field. However, current studies are targeting particles administration directly into the tumor site.
Indeed, in vivo TMMEP studies remain currently limited to initial observations. To confirm the good preliminary results obtained in vivo, new study should be done, facing the problematic of particles injection and diffusion in the tumor. Moreover, three-dimensional cell culture models, as recently presented by (Lunov et al., 2019),51 as well as in our recent studies,101,102 can advantageously mimic tumoral in vivo conditions, allowing to vary numerous relevant parameters.
The in vitro studies, although presenting highly variable experimental conditions, have allowed to test the behavior and efficiency of various types of particles. The particles of anisotropic shape turned out to exhibit advantages in different aspects of this approach. Favorable for the potential circulation in the blood stream, the anisotropic particles are likewise more efficient for converting magnetic forces or torques into mechanical effects on cells, leading to an efficient magneto-mechanical actuation. Furthermore, in vitro studies shows that depending on the particles anisotropic shapes – discs or elongated cylinders such as nanorods – , the occurrence of cell death pathway differs. Perturbations of the cellular membrane, the lysosome or the cytoskeleton in most cases led to apoptotic cell death pathway. However, necrosis without apoptosis was also reported. It can be noted that apoptosis is systematically reported in studies involving disk-shape particles, whereas nanorods or nanowires could be more destructive and potentially cause necrosis cell death. Spherical particles, such as SPIONs, likewise led to cancer cells destruction via apoptosis, as reported in two-thirds of studies using them. All these types of particles could however form small anisotropic chains or clusters when they are submitted to an applied magnetic field, leading to various shapes of magnetic microstructures acting on the biological cells.
Moreover, in terms of particles composition, ferromagnetic particles, since presenting higher magnetization than iron oxide nanoparticles, will exert larger forces or torques for a given magnetic volume. It may be noted that for any particle shape or composition, the cytotoxicity risks will have to be systematically assessed, since each type of particle has its own degre of toxicity, including SPIONS through ROS. Coating the particles with a biocompatible layer may be necessary, also allowing the bio-functionalization of particles. The particles dispersion capability have been demonstrated for SPIONS as well as for ferromagnetic vortex particles, and for SAF particles under a threshold of magnetic susceptibility. Concerning the source of magnetic field, Halbach cylinder turned out to represent the magnetic field set-up providing the larger magnetic field remaining uniform in a rather large space. However for potential clinical applications, manufacturing Halbach cylinders with appropriate dimensions for treating the human body may be challenging. Other magnetic sources such as magnetic stirrer could thus be appropriate for TMMEP, despite a less uniform magnetic field.
The analysis of such studies firstly shows the growing interest in this magneto-mechanical approach, launched only ten years ago, initially published by Kim et al., 2010.22 The review reveals the great diversity of experimental conditions, and should yield a better assessments of certain cancer cell death parameters. The various type of magnetic micro–nanoparticles used in the different studies – their shapes, sizes, compositions, the resulting magnetic states and properties – the non-agglomeration requirements and the advantages of magnetic anisotropy for an efficient mechanical actuation, have been detailed. Likewise, the various available sources of alternative magnetic fields or field gradient have been presented.
In summary, TMMEP (cancer treatment by magneto-mechanical effect of particles) is based on a mechanical impact on cells induced by magnetic particles movement. This promising technique for cancer therapy show interesting effects on cancer cells in vitro. Nevertheless, more studies are needed to clearly identify the best parameters. A better understanding of cellular mechanisms involved could help to trigger specific pathways leading to cellular death. The induction of apoptosis – cell death mode particularly sought, minimizing adverse effects such as inflammation – is highlighted and its rate quantitatively evaluated in various studies.
Main advantages of TMMEP compared to other cancer therapy such as magnetic hyperthermia, surgery or pharmacotherapies, could be less side effects and low invasiveness of the technique. TMMEP method is reported as appropriate for sparing the surrounding healthy cells, since the magneto-mechanical vibration applied on or within the targeted cancer cells remains highly local, in contrast with methods using heat which tends to diffuse into the neighboring tissues.22,24
Although these first trials of TMMEP treatments on cancer cells have yet to be deepened, the method could allow great improvements in future cancer treatments, and a hope for treating cancer with very poor prognosis as glioblastoma, for instance. Investigations on cancer cells destruction through magnetically actuated microparticles vibrating at low frequency, are finally of great interest for future cancer therapy, while remaining a big challenge. They will have to be pursued and deepened, in an effort to develop, on longer term, targeted cancer treatments with reduced side effects.
6. Conclusion–perspectives
The field of magneto-mechanical anti-cancer therapies has exponentially growth for the last 5 years, paving the foundation for new therapies in oncology. The main efficacy demonstration was done in vitro, using highly heterogeneous magnetic-responsive nanoparticles and magnetic stimulation. Beside the inaugural thermal effect, the demonstration that mechanical stimulation can modulate the cell biology of cancer is further comforted by the recent demonstration of mechano-transduction pathways. These pathways as well as the connected physico-mechanic properties of the tissues are probably as important for the physiological tissue homeostasis than the classical molecular pathways governing the initiation and promotion of cancer. Only a few in vivo investigations have been done, contrasting with the number of in vitro studies. In vivo strategies are confronted by the bottleneck of tissue delivery that is very poor after intravenous injection. Intratumor delivery is a growing alternative in nanomedicine that will be probably became the first strategy, increasing the efficacy of local nanoparticle delivery as well as it decreases the potential systemic side effects. Anticipating from the beginning of these investigations the biocompatibility of the nanoparticle is mandatory. Moreover, it will be also mandatory to decipher the physical properties of the targeted tissue, such as stiffness and intra-tissular pressure that could modulate the efficacy of magneto-mechanical therapy. Several imaging methodologies have been developed for that such as ultrasound stiffness imaging. A mechanical dosimetry mapping of the tissue should provide the opportunity of a real mechanical therapy personalization.Both in vitro and in vivo studies in the field of magneto-mechanical therapies of cancer pave the way for a real renewing of cancer therapies, responding to the therapeutical resistances observed in the field of chemotherapy and targeted molecular and cellular therapies. Translating physics and nano-magnetism at the beside will need a strong interdisciplinarity associating synergistically physician, biologists and physicists.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by funding from the European Union's H2020 ERA-Net EuroNanoMed II program Nanoviber Project – 16-ENM2-0008-02.References
- Q. A. Pankhurst, J. Connolly, S. K. Jones and J. P. Dobson, Applications of magnetic nanoparticles in biomedicine, J. Phys. D: Appl. Phys., 2003, 36, 167–181 CrossRef.
- Y. Cheng, R. A. Morshed, B. Auffinger, A. L. Tobias and M. S. Lesniak, Multifunctional nanoparticles for brain tumor imaging and therapy, Adv. Drug Delivery Rev., 2014, 66, 42–57 CrossRef CAS PubMed.
- Z. R. Stephen, F. M. Kievit and M. Zhang, Magnetite nanoparticles for medical MR imaging, Mater. Today, 2011, 14, 330–338 CrossRef CAS PubMed.
- R. Jin, B. Lin, D. Li and H. Ai, Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications, Curr. Opin. Pharmacol., 2014, 18, 18–27 CrossRef CAS PubMed.
- M. Arruebo, R. Fernández-pacheco, M. R. Ibarra and J. Santamaría, Magnetic nanoparticles for drug delivery, Nano Today, 2007, 2, 22–32 CrossRef.
- V. V. Mody, A. Cox, S. Shah, A. Singh, W. Bevins and H. Parihar, Magnetic nanoparticle drug delivery systems for targeting tumor, Appl. Nanosci., 2014, 4, 385–392 CrossRef CAS.
- C. Sun, J. S. H. Lee and M. Zhang, Magnetic nanoparticles in MR imaging and drug delivery, Adv. Drug Delivery Rev., 2008, 60, 1252–1265 CrossRef CAS PubMed.
- O. L. Gobbo, K. Sjaastad, M. W. Radomski, Y. Volkov and A. Prina-Mello, Magnetic nanoparticles in cancer theranostics, Theranostics, 2015, 5, 1249–1263 CrossRef CAS PubMed.
- Y. Gao, J. Lim, S.-H. Teoh and C. Xu, Emerging translational research on magnetic nanoparticles for regenerative medicine, Chem. Soc. Rev., 2015, 44, 6306–6329 RSC.
- Q. Zhang, T. Yin, R. Xu, W. Gao, H. Zhao, J. G. Shapter, K. Wang, Y. Shen, P. Huang, G. Gao, Y. Wu and D. Cui, Large-scale immuno-magnetic cell sorting of T cells based on a self-designed high-throughput system for potential clinical application, Nanoscale, 2017, 9, 13592–13599 RSC.
- S. H. Cartmell, A. Keramane, G. R. Kirkham, S. B. Verschueren, J. L. Magnay, A. J. El Haj and J. Dobson, Use of magnetic particles to apply mechanical forces for bone tissue engineering purposes, J. Phys.: Conf. Ser., 2005, 17, 77–80 CrossRef CAS.
- J. L. Corchero and A. Villaverde, Biomedical applications of distally controlled magnetic nanoparticles, Trends Biotechnol., 2009, 27, 468–476 CrossRef CAS PubMed.
- C. Sanchez, D. El Hajj Diab, V. Connord, P. Clerc, E. Meunier, B. Pipy, B. Payré, R. P. Tan, M. Gougeon, J. Carrey, V. Gigoux and D. Fourmy, Targeting a G-protein-coupled receptor overexpressed in endocrine tumors by magnetic nanoparticles to induce cell death, ACS Nano, 2014, 8, 1350–1363 CrossRef CAS.
- L. Kafrouni and O. Savadogo, Recent progress on magnetic nanoparticles for magnetic hyperthermia, Prog. Biomater., 2016, 5, 147–160 CrossRef CAS PubMed.
- S. Dutz and R. Hergt, Magnetic particle hyperthermia – a promising tumour therapy?, Nanotechnology, 2014, 25, 1–28 CrossRef PubMed.
- E. A. Vitol, V. Novosad and E. A. Rozhkova, Microfabricated magnetic structures for future medicine: from sensors to cell actuators, Nanomedicine, 2012, 7, 1611–1624 CrossRef CAS PubMed.
- R. J. Mannix, S. Kumar, F. Cassiola, M. Montoya-Zavala, E. Feinstein, M. Prentiss and D. E. Ingber, Nanomagnetic actuation of receptor-mediated signal transduction, Nat. Nanotechnol., 2008, 3, 36–40 CrossRef CAS PubMed.
- S. Hughes, S. McBain, J. P. Dobson and A. J. El Haj, Selective activation of mechanosensitive ion channels using magnetic particles, J. R. Soc., Interface, 2008, 5, 855–863 CrossRef CAS PubMed.
- A. O. Fung, V. Kapadia, E. Pierstorff, D. Ho and Y. Chen, Induction of cell death by magnetic actuation of nickel nanowires internalized by fibroblasts, J. Phys. Chem. C, 2008, 112, 15085–15088 CrossRef CAS.
- V. Connord, P. Clerc, N. Hallali, D. El Hajj Diab, D. Fourmy, V. Gigoux and J. Carrey, Real-time analysis of magnetic hyperthermia experiments on living cells under a confocal microscope, Small, 2015, 11, 2437–2445 CrossRef CAS.
- M. Domenech, I. Marrero-Berrios, M. Torres-Lugo and C. Rinaldi, Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields, ACS Nano, 2013, 7, 5091–5101 CrossRef CAS PubMed.
- D.-H. Kim, E. A. Rozhkova, I. V Ulasov, S. D. Bader, T. Rajh, M. S. Lesniak and V. Novosad, Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction, Nat. Mater., 2010, 9, 165–171 CrossRef CAS PubMed.
- V. Novosad and E. A. Rozhkova, Ferromagnets-Based Multifunctional Nanoplatform for Targeted Cancer Therapy, in Biomedical Engineering, Trends in Materials Science, 2011, pp. 425–444 Search PubMed.
- M. Goiriena-Goikoetxea, D. Muñoz, I. Orue, M. L. Fernández-Gubieda, J. Bokor, A. Muela and A. García-Arribas, Disk-shaped magnetic particles for cancer therapy, Appl. Phys. Rev., 2020, 7, 011306 Search PubMed.
- M. Chen, J. Wu, P. Ning, J. Wang, Z. Ma, L. Huang, G. R. Plaza, Y. Shen, C. Xu, Y. Han, M. S. Lesniak, Z. Liu and Y. Cheng, Remote Control of Mechanical Forces via Mitochondrial-Targeted Magnetic Nanospinners for Efficient Cancer Treatment, Small, 2020, 16, 1905424 CrossRef CAS PubMed.
- N. Maniotis, A. Makridis, E. Myrovali, A. Theopoulos, T. Samaras and M. Angelakeris, Magneto-mechanical action of multimodal field configurations on magnetic nanoparticle environments, J. Magn. Magn. Mater., 2019, 470, 6–11 CrossRef CAS.
- R. Mansell, T. Vemulkar, D. C. M. C. Petit, Y. Cheng, J. Murphy, M. S. Lesniak and R. P. Cowburn, Magnetic particles with perpendicular anisotropy for mechanical cancer cell destruction, Sci. Rep., 2017, 7, 1–7 CrossRef CAS PubMed.
- H. Ye, Z. Shen, L. Yu, M. Wei and Y. Li, Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine, Proc. R. Soc. A, 2018, 474, 1–24 CrossRef PubMed.
- B. Wang, C. Bienvenu, J. Mendez-Garza, P. Lançon, A. Madeira, P. Vierling, C. Di Giorgio and G. Bossis, Necrosis of HepG2 cancer cells induced by the vibration of magnetic particles, J. Magn. Magn. Mater., 2013, 344, 193–201 CrossRef CAS.
- Y. Shen, C. Wu, T. Q. P. Uyeda, G. R. Plaza, B. Liu, Y. Han, M. S. Lesniak and Y. Cheng, Elongated nanoparticle aggregates in cancer cells for mechanical destruction with low frequency rotating magnetic field, Theranostics, 2017, 7, 1735–1748 CrossRef CAS.
- D. Liu, L. Wang, Z. Wang and A. Cuschieri, Magnetoporation and magnetolysis of cancer cells via carbon nanotubes induced by rotating magnetic fields, Nano Lett., 2012, 12, 5117–5121 CrossRef CAS.
- D. Cheng, X. Li, G. Zhang and H. Shi, Morphological effect of oscillating magnetic nanoparticles in killing tumor cells, Nanoscale Res. Lett., 2014, 9, 1–8 CrossRef CAS.
- M. F. Contreras, R. Sougrat, A. Zaher, T. Ravasi and J. Kosel, Non-chemotoxic induction of cancer cell death using magnetic nanowires, Int. J. Nanomed., 2015, 10, 2141–2153 CrossRef CAS PubMed.
- D. Kilinc, A. Lesniak, S. A. Rashdan, D. Gandhi, A. Blasiak, P. C. Fannin, A. von Kriegsheim, W. Kolch and G. U. Lee, Mechanochemical Stimulation of MCF7 Cells with Rod-Shaped Fe–Au Janus Particles Induces Cell Death Through Paradoxical Hyperactivation of ERK, Adv. Healthcare Mater., 2015, 4, 395–404 CrossRef CAS PubMed.
- A. I. Martínez-Banderas, A. Aires, F. J. Teran, J. E. Perez, J. F. Cadenas, N. Alsharif, T. Ravasi, A. L. Cortajarena and J. Kosel, Functionalized magnetic nanowires for chemical and magneto-mechanical induction of cancer cell death, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- D. W. Wong, W. L. Gan, N. Liu and W. S. Lew, Magneto-actuated cell apoptosis by biaxial pulsed magnetic field, Sci. Rep., 2017, 7, 1–8 CrossRef CAS PubMed.
- Y. Cheng, M. E. Muroski, D. C. M. C. Petit, R. Mansell, T. Vemulkar, R. A. Morshed, Y. Han, I. V. Balyasnikova, C. M. Horbinski, X. Huang, L. Zhang, R. P. Cowburn and M. S. Lesniak, Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma, J. Controlled Release, 2016, 223, 75–84 CrossRef CAS PubMed.
- S. Leulmi, X. Chauchet, M. Morcrette, G. Ortiz, H. Joisten, P. Sabon, T. Livache, Y. Hou, M. Carrière, S. Lequien and B. Dieny, Triggering the apoptosis of targeted human renal cancer cells by the vibration of anisotropic magnetic particles attached to the cell membrane, Nanoscale, 2015, 7, 15904–15914 RSC.
- M. E. Muroski, R. A. Morshed, Y. Cheng, T. Vemulkar, R. Mansell, Y. Han, L. Zhang, K. S. Aboody, R. P. Cowburn and M. S. Lesniak, Controlled payload release by magnetic field triggered neural stem cell destruction for malignant glioma treatment, PLoS One, 2016, 1–12 Search PubMed.
- T. N. Zamay, G. S. Zamay, I. V Belyanina, S. S. Zamay, V. V Denisenko, O. S. Kolovskaya, T. I. Ivanchenko, V. L. Grigorieva, I. V Garanzha, D. V Veprintsev, Y. E. Glazyrin, A. V Shabanov, V. Y. Prinz, V. A. Seleznev, A. E. Sokolov, V. S. Propenko, P. D. Kim, A. Gargaun, M. V Berezovski and A. S. Zamay, Noninvasive microsurgery using aptamer-functionalized magnetic microdisks for tumor cell eradication, Nucleic Acid Ther., 2017, 27, 105–114 CrossRef CAS PubMed.
- S.-H. Hu and X. Gao, Nanocomposites with Spatially Separated Functionalities for Combined Imaging and Magnetolytic Therapy, J. Am. Chem. Soc., 2010, 132, 7234–7237 CrossRef CAS PubMed.
- M. H. Cho, E. J. Lee, M. Son, J. H. Lee, D. Yoo, J. W. Kim, S. W. Park, J. S. Shin and J. Cheon, A magnetic switch for the control of cell death signalling in in vitro and in vivo systems, Nat. Mater., 2012, 11, 1038–1043 CrossRef CAS PubMed.
- E. Zhang, M. F. Kircher, M. Koch, L. Eliasson, S. N. Goldberg and E. Renström, Dynamic Magnetic Fields Remote-Control Apoptosis via Nanoparticle Rotation, ACS Nano, 2014, 8, 3192–3201 CrossRef CAS.
- A. M. Master, P. N. Williams, N. Pothayee, N. Pothayee, R. Zhang, H. M. Vishwasrao, Y. I. Golovin, J. S. Riffle, M. Sokolsky and A. V. Kabanov, Remote actuation of magnetic nanoparticles for cancer cell selective treatment through cytoskeletal disruption, Sci. Rep., 2016, 6, 1–13 CrossRef.
- F. Wo, R. Xu, Y. Shao, Z. Zhang, M. Chu, D. Shi and S. Liu, A multimodal system with synergistic effects of magneto-mechanical, photothermal, photodynamic and chemo therapies of cancer in graphene-quantum dot-coated hollow magnetic nanospheres, Theranostics, 2016, 6, 485–500 CrossRef CAS.
- H. Ju, Y. Cui, Z. Chen, Q. Fu, M. Sun and Y. Zhou, Effects of combined delivery of extremely low frequency electromagnetic field and magnetic Fe3O4 nanoparticles on hepatic cell lines, Am. J. Transl. Res., 2016, 8, 1838–1847 CAS.
- R. Brossel, A. Yahi, S. David, L. Moreno Velasquez and J.-M. Guinebretière, Mechanical Signals Inhibit Growth of a Grafted Tumor In Vivo: Proof of Concept, PLoS One, 2016, 11, e0152885 CrossRef PubMed.
- S. Hapuarachchige, Y. Kato, E. J. Ngen, B. Smith, M. Delannoy and D. Artemov, Non-temperature induced effects of magnetized iron oxide nanoparticles in alternating magnetic field in cancer cells, PLoS One, 2016, 11, 1–12 CrossRef PubMed.
- A. Vegerhof, E. A. Barnoy, M. Motiei, D. Malka, Y. Danan, Z. Zalevsky and R. Popovtzer, Targeted magnetic nanoparticles for mechanical lysis of tumor cells by low-amplitude alternating magnetic field, Materials, 2016, 9, 1–12 CrossRef PubMed.
- W. Li, Y. Liu, Z. Qian and Y. Yang, Evaluation of Tumor Treatment of Magnetic Nanoparticles Driven by Extremely Low Frequency Magnetic Field, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- O. Lunov, M. Uzhytchak, B. Smolková, M. Lunova, M. Jirsa, N. M. Dempsey, A. L. Dias, M. Bonfim, M. Hof, P. Jurkiewicz, Y. Petrenko, Š. Kubinová and A. Dejneka, Remote actuation of apoptosis in liver cancer cells via magneto-mechanical modulation of iron oxide nanoparticles, Cancers, 2019, 11, 1–20 CrossRef PubMed.
- H. Chiriac, E. Radu, M. Țibu, G. Stoian, G. Ababei, L. Lăbușcă, D.-D. Herea and N. Lupu, Fe–Cr–Nb–B ferromagnetic particles with shape anisotropy for cancer cell destruction by magneto-mechanical actuation, Sci. Rep., 2018, 8, 1–9 CrossRef CAS PubMed.
- D. Ling and T. Hyeon, Chemical design of biocompatible iron oxide nanoparticles for medical applications, Small, 2013, 9, 1450–1466 CrossRef CAS PubMed.
- M. Samadishadlou, M. Farshbaf, N. Annabi, T. Kavetskyy, R. Khalilov, S. Saghfi, A. Akbarzadeh and S. Mousavi, Magnetic carbon nanotubes: preparation, physical properties, and applications in biomedicine, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1314–1330 CrossRef CAS PubMed.
- S. Leulmi, H. Joisten, T. Dietsch, C. Iss, M. Morcrette, S. Auffret, P. Sabon and B. Dieny, Comparison of dispersion and actuation properties of vortex and synthetic antiferromagnetic particles for biotechnological applications, Appl. Phys. Lett., 2013, 103, 8–13 CrossRef.
- M. Ermolli, C. Menné, G. Pozzi, M. ángel Serra and L. A. Clerici, Nickel, cobalt and chromium-induced cytotoxicity and intracellular accumulation in human HaCaT keratinocytes, Toxicology, 2001, 159, 23–31 CrossRef CAS.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, The golden age: gold nanoparticles for biomedicine, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- R. Shukla, V. Bansal, M. Chaudhary, A. Basu, R. R. Bhonde and M. Sastry, Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview, Langmuir, 2005, 21, 10644–10654 CrossRef CAS PubMed.
- H. Hartmann and R. Krastev, Biofunctionalization of surfaces using polyelectrolyte multilayers, BioNanoMaterials, 2017, 20160015 Search PubMed , 12pp.
- I. Bodhinayake, M. Ottenhausen and J. A. Boockvar, Targeting a heterogeneous tumor: The promise of the interleukin-13 receptor α2, Neurosurgery, 2014, 75, 18–19 CrossRef PubMed.
- C. G. Hadjipanayis, R. Machaidze, M. Kaluzova, L. Wang, A. J. Schuette, H. Chen, X. Wu and H. Mao, EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma, Cancer Res., 2010, 70, 6303–6312 CrossRef CAS.
- E. Prats-Alfonso and F. Albericio, Functionalization of gold surfaces: recent developments and applications, J. Mater. Sci., 2011, 46, 7643–7648 CrossRef CAS.
- P. Decuzzi, B. Godin, T. Tanaka, S. Y. Lee, C. Chiappini, X. Liu and M. Ferrari, Size and shape effects in the biodistribution of intravascularly injected particles, J. Controlled Release, 2010, 141, 320–327 CrossRef CAS.
- A. Albanese, P. S. Tang and W. C. W. Chan, The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS.
- H. Ye, Z. Shen and Y. Li, Computational modeling of magnetic particle margination within blood flow through LAMMPS, Comput. Mech., 2018, 62, 457–476 CrossRef.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater., 2016, 1, 1–12 Search PubMed.
- Y. Geng, P. Dalhaimer, S. Cai, R. Tsai, M. Tewari, T. Minko and D. E. Discher, Shape effects of filaments versus spherical particles in flow and drug delivery, Nat. Nanotechnol., 2007, 2, 249–255 CrossRef CAS PubMed.
- E. Carboni, K. Tschudi, J. Nam, X. Lu and A. W. K. Ma, Particle margination and its implications on intravenous anticancer drug delivery, AAPS PharmSciTech, 2014, 15, 762–771 CrossRef CAS PubMed.
- F. Gentile, A. Curcio, C. Indolfi, M. Ferrari and P. Decuzzi, The margination propensity of spherical particles for vascular targeting in the microcirculation, J. Nanobiotechnol., 2008, 6, 1–9 CrossRef PubMed.
- V. P. Chauhan, Z. Popović, O. Chen, J. Cui, D. Fukumura, M. G. Bawendi and R. K. Jain, Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration, Angew. Chem., Int. Ed., 2011, 50, 11417–11420 CrossRef CAS PubMed.
- S. K. Hobbs, W. L. Monsky, F. Yuan, W. G. Roberts, L. Griffith, V. P. Torchilin and R. K. Jain, Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 4607–4612 CrossRef CAS PubMed.
- Q. Li, C. W. Kartikowati, S. Horie, T. Ogi, T. Iwaki and K. Okuyama, Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles, Sci. Rep., 2017, 7, 1–4 CrossRef PubMed.
- N. H. Hai, N. H. Luong, N. Chau and N. Q. Tai, Preparation of magnetic nanoparticles embedded in polystyrene microspheres, J. Phys.: Conf. Ser., 2009, 187, 1–6 Search PubMed.
- K. Hayashi, M. Nakamura, W. Sakamoto, T. Yogo, H. Miki, S. Ozaki, M. Abe, T. Matsumoto and K. Ishimura, Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment, Theranostics, 2013, 3, 366–376 CrossRef CAS PubMed.
- S. Kralj and D. Makovec, Magnetic Assembly of Superparamagnetic Iron Oxide Nanoparticle Clusters into Nanochains and Nanobundles, ACS Nano, 2015, 9, 9700–9707 CrossRef CAS PubMed.
- H. Joisten, T. Courcier, P. Balint, P. Sabon, J. Faure-Vincent, S. Auffret and B. Dieny, Self-polarization phenomenon and control of dispersion of synthetic antiferromagnetic nanoparticles for biological applications, Appl. Phys. Lett., 2010, 97, 2–4 CrossRef.
- K. Y. Guslienko, V. Novosad, Y. Otani, H. Shima and K. Fukamichi, Field evolution of magnetic vortex state in ferromagnetic disks, Appl. Phys. Lett., 2001, 78, 3848–3850 CrossRef CAS.
- W. Hu, R. J. Wilson, A. Koh, A. Fu, A. Z. Faranesh, C. M. Earhart, S. J. Osterfeld, S. J. Han, L. Xu, S. Guccione, R. Sinclair and S. X. Wang, High-moment antiferromagnetic nanoparticles with tunable magnetic properties, Adv. Mater., 2008, 20, 1479–1483 CrossRef CAS.
- T. Vemulkar, R. Mansell, D. C. M. C. Petit, R. P. Cowburn and M. S. Lesniak, Highly tunable perpendicularly magnetized synthetic antiferromagnets for biotechnology applications, Appl. Phys. Lett., 2015, 107, 012403 CrossRef CAS PubMed.
- E. A. Rozhkova, V. Novosad, D.-H. Kim, J. Pearson, R. Divan, T. Rajh and S. D. Bader, Ferromagnetic microdisks as carriers for biomedical applications, J. Appl. Phys., 2009, 105, 58–61 CrossRef.
- M. Goiriena-Goikoetxea, A. García-Arribas, M. Rouco, A. V. Svalov and J. M. Barandiaran, High-yield fabrication of 60 nm permalloy nanodiscs in well-defined magnetic vortex state for biomedical applications, Nanotechnology, 2016, 27, 175302 CrossRef CAS PubMed.
- Y. P. Ivanov, M. Vázquez and O. Chubykalo-Fesenko, Magnetic reversal modes in cylindrical nanowires, J. Phys. D: Appl. Phys., 2013, 46, 11pp Search PubMed.
- H. Joisten, A. Truong, S. Ponomareva, C. Naud, R. Morel, Y. Hou, I. Joumard, S. Auffret, P. Sabon and B. Dieny, Optical response of magnetically actuated biocompatible membranes, Nanoscale, 2019, 11, 10667–10683 RSC.
- M. Morcrette, Micro et nanoparticules pour des applications biotechnologiques: fabrication de nanoparticules par copolymère dibloc pour l’imagerie médicale; destruction de cellules cancéreuses par vibrations magnéto- mécaniques de microparticules magnétiques, PhD thesis, Univ. Grenoble Alpes, 2015, http://https://www.theses.fr/2015GREAY052 Search PubMed.
- Y. Chen, L. Ju, M. Rushdi, C. Ge and C. Zhu, Receptor-mediated cell mechanosensing, Mol. Biol. Cell, 2017, 3134–3155 CrossRef CAS PubMed.
- L. Galluzzi, I. Vitale, S. A. Aaronson, J. M. Abrams, D. Adam, P. Agostinis, E. S. Alnemri, L. Altucci, I. Amelio, D. W. Andrews, M. Annicchiarico-Petruzzelli, A. V. Antonov, E. Arama, E. H. Baehrecke, N. A. Barlev, N. G. Bazan, F. Bernassola, M. J. M. Bertrand, K. Bianchi, M. V. Blagosklonny, K. Blomgren, C. Borner, P. Boya, C. Brenner, M. Campanella, E. Candi, D. Carmona-Gutierrez, F. Cecconi, F. K. M. Chan, N. S. Chandel, E. H. Cheng, J. E. Chipuk, J. A. Cidlowski, A. Ciechanover, G. M. Cohen, M. Conrad, J. R. Cubillos-Ruiz, P. E. Czabotar, V. D'Angiolella, T. M. Dawson, V. L. Dawson, V. De Laurenzi, R. De Maria, K. M. Debatin, R. J. Deberardinis, M. Deshmukh, N. Di Daniele, F. Di Virgilio, V. M. Dixit, S. J. Dixon, C. S. Duckett, B. D. Dynlacht, W. S. El-Deiry, J. W. Elrod, G. M. Fimia, S. Fulda, A. J. García-Sáez, A. D. Garg, C. Garrido, E. Gavathiotis, P. Golstein, E. Gottlieb, D. R. Green, L. A. Greene, H. Gronemeyer, A. Gross, G. Hajnoczky, J. M. Hardwick, I. S. Harris, M. O. Hengartner, C. Hetz, H. Ichijo, M. Jäättelä, B. Joseph, P. J. Jost, P. P. Juin, W. J. Kaiser, M. Karin, T. Kaufmann, O. Kepp, A. Kimchi, R. N. Kitsis, D. J. Klionsky, R. A. Knight, S. Kumar, S. W. Lee, J. J. Lemasters, B. Levine, A. Linkermann, S. A. Lipton, R. A. Lockshin, C. López-Otín, S. W. Lowe, T. Luedde, E. Lugli, M. MacFarlane, F. Madeo, M. Malewicz, W. Malorni, G. Manic, J. C. Marine, S. J. Martin, J. C. Martinou, J. P. Medema, P. Mehlen, P. Meier, S. Melino, E. A. Miao, J. D. Molkentin, U. M. Moll, C. Muñoz-Pinedo, S. Nagata, G. Nuñez, A. Oberst, M. Oren, M. Overholtzer, M. Pagano, T. Panaretakis, M. Pasparakis, J. M. Penninger, D. M. Pereira, S. Pervaiz, M. E. Peter, M. Piacentini, P. Pinton, J. H. M. Prehn, H. Puthalakath, G. A. Rabinovich, M. Rehm, R. Rizzuto, C. M. P. Rodrigues, D. C. Rubinsztein, T. Rudel, K. M. Ryan, E. Sayan, L. Scorrano, F. Shao, Y. Shi, J. Silke, H. U. Simon, A. Sistigu, B. R. Stockwell, A. Strasser, G. Szabadkai, S. W. G. Tait, D. Tang, N. Tavernarakis, A. Thorburn, Y. Tsujimoto, B. Turk, T. Vanden Berghe, P. Vandenabeele, M. G. Vander Heiden, A. Villunger, H. W. Virgin, K. H. Vousden, D. Vucic, E. F. Wagner, H. Walczak, D. Wallach, Y. Wang, J. A. Wells, W. Wood, J. Yuan, Z. Zakeri, B. Zhivotovsky, L. Zitvogel, G. Melino and G. Kroemer, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018, Cell Death Differ., 2018, 25, 486–541 CrossRef PubMed.
- I. K. H. Poon, C. D. Lucas, A. G. Rossi and K. S. Ravichandran, Apoptotic cell clearance: basic biology and therapeutic potential, Nat. Rev. Immunol., 2014, 14, 166–180 CrossRef CAS PubMed.
- Z. Jin and W. S. El-Deiry, Overview of cell death signaling pathways, Cancer Biol. Ther., 2005, 4, 147–171 CrossRef PubMed.
- G. Liu, C. Wu, Y. Wu and Y. Zhao, Phagocytosis of Apoptotic Cells and Immune Regulation, Scand. J. Immunol., 2006, 64, 1–9 CrossRef CAS PubMed.
- M. Desouza, P. W. Gunning and J. R. Stehn, The actin cytoskeleton as a sensor and mediator of apoptosis, Bioarchitecture, 2012, 2, 75–87 CrossRef PubMed.
- R. M. Mohammada, I. Muqbil, L. Lowe, C. Yedjou, H.-Y. Hsu, L.-T. Lin, M. D. Siegelin, C. Fimognari, N. B. Kumar, Q. P. Dou, H. Yang, A. K. Samadi, G. L. Russo, C. Spagnuolo, S. K. Ray, M. Chakrabarti, J. D. Morre, H. M. Coley, K. Honoki, H. Fujii, A. G. Georgakila, A. Amedei, E. Niccolai, A. Amin, S. S. Ashraf, W. G. Helferich, X. Yang, C. S. Boosani, G. Guha, D. Bhakta, M. R. Ciriolo, K. Aquilano, S. Chen, S. I. Mohammed, W. N. Keith, A. Bilsland, D. Halicka, S. Nowsheen and A. S. Azmi, Broad targeting of resistance to apoptosis in cancer, Semin. Cancer Biol., 2015, 35, 1–63 CrossRef PubMed.
- A. Kroll, M. H. Pillukat, D. Hahn and J. Schnekenburger, Interference of engineered nanoparticles with in vitro toxicity assays, Arch. Toxicol., 2012, 86, 1123–1136 CrossRef CAS PubMed.
- K. J. Ong, T. J. MacCormack, R. J. Clark, J. D. Ede, V. A. Ortega, L. C. Felix, M. K. M. Dang, G. Ma, H. Fenniri, J. G. C. Veinot and G. G. Goss, Widespread Nanoparticle-Assay Interference: Implications for Nanotoxicity Testing, PLoS One, 2014, 9, e90650 1–9 Search PubMed.
- N. Kavčič, K. Pegan and B. Turk, Lysosomes in programmed cell death pathways: from initiators to amplifiers, Biol. Chem., 2017, 398, 289–301 Search PubMed.
- A. M. Villamil Giraldo, H. Appelqvist, T. Ederth and K. Öllinger, Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death, Biochem. Soc. Trans., 2014, 42, 1460–1464 CrossRef CAS.
- C. W. Gourlay and K. R. Ayscough, The actin cytoskeleton: a key regulator of apoptosis and ageing?, Nat. Rev. Mol. Cell Biol., 2005, 6, 583–589 CrossRef CAS PubMed.
- J. P. Dobson, Remote control of cellular behaviour with magnetic nanoparticles, Nat. Nanotechnol., 2008, 3, 139–143 CrossRef CAS.
- F. Broders-Bondon, T. H. N. Ho-Bouldoires, M. E. Fernandez-Sanchez and E. Farge, Mechanotransduction in tumor progression: the dark side of the force, J. Cell Biol., 2018, 217, 1571–1587 CrossRef CAS PubMed.
- D. D. Vo, R. M. Prins, J. L. Begley, T. R. Donahue, L. F. Morris, K. W. Bruhn, P. de la Rocha, M.-Y. Yang, S. Mok, H. J. Garban, N. Craft, J. S. Economou, F. M. Marincola, E. Wang and A. Ribas, Enhanced Antitumor Activity Induced by Adoptive T-Cell Transfer and Adjunctive Use of the Histone Deacetylase Inhibitor LAQ824, Cancer Res., 2009, 69, 8693–8699 CrossRef CAS PubMed.
- T. Klei, F. Souza, M. P. Nucci, J. B. Mamani, R. Silva, D. Maciely, C. Fantacini, L. E. B. De, V. Picanc, D. T. Covas, E. L. Vidoto, T. K. Felix Souza, M. P. Nucci, J. B. Mamani, H. Rodrigues da Silva, D. M. Carvalho Fantacini, L. E. Botelho de Souza, V. Picanço-Castro, D. T. Covas, E. L. Vidoto, A. Tannús and L. F. Gamarra, Image and motor behavior for monitoring tumor growth in C6 glioma model, PLoS One, 2018, 13, 1–21 Search PubMed.
- C. Naud, Particules magnétiques pour le traitement du cancer par effet magnéto-mécanique, application au glioblastome, PhD thesis, Université Grenoble Alpes, 2019, http://www.theses.fr/2019GREAS005 Search PubMed.
- C. Naud, A 3D spheroid-based model for preclinical evaluation of treatment by magneto mechanical effect of particles in cancer therapy, to be publ.
- S. Rebouissou, J. Zucman-Rossi, R. Moreau, Z. Qiu and L. Hui, Note of caution: contaminations of hepatocellular cell lines, J. Hepatol., 2017, 67, 896–897 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |