Open Access Article
Open Access ArticleL-Cysteine-mediated modulation of copper trafficking in prostate cancer cells: an in vitro and in vivo investigation with 64Cu and 64Cu-PET†
Joanna J.
Bartnicka
 ,
Fahad
Al-salemee
,
Fahad
Al-salemee
 ,
George
Firth
,
George
Firth
 and
Philip J.
Blower
and
Philip J.
Blower
 *
*
School of Biomedical Engineering & Imaging Sciences, King's College London, St Thomas’ Hospital, London, SE1 7EH, UK. E-mail: philip.blower@kcl.ac.uk
First published on 9th September 2020
Abstract
Copper imbalance is implicated in many diseases, including cancer. Copper in blood is mainly transported by carrier proteins but a small fraction is bound to low molecular weight species, possibly amino acids. Their roles in cellular copper delivery are unknown. Our aim was to test whether accumulation of 64Cu into cancer-derived cells can be influenced by copper-binding serum amino acids. In vitro cellular accumulation of 64Cu was measured in Hank's Balanced Salt Solution in the presence of 100 μM L-histidine, L-methionine, L-cysteine and L-threonine. L-Cysteine markedly increased 64Cu accumulation and retention in DU145, PC3 and SK-OV-3 cells, while some other cell lines did not show an effect. This effect was not due to 64Cu delivery in the form of a 64Cu–cysteine complex, nor to reduction of 64Cu(II) to 64Cu(I) by L-cysteine. Pre-incubation of cells with L-cysteine increased 64Cu accumulation, even if L-cysteine was removed from HBSS before 64Cu was added. The effect of L-cysteine on 64Cu accumulation was not mediated by increased glutathione synthesis. Despite the demonstrable in vitro effect, pre-injection of L-cysteine precursor N-acetyl-cysteine (NAC) in vivo did not enhance 64Cu delivery to DU145 xenografts in mice. Instead, it decreased 64Cu accumulation in the DU145 tumour and in brain, as assessed by PET imaging. We conclude that 64Cu is not delivered to DU145 cancer cells in vitro as a complex with amino acids but its cellular accumulation is enhanced by L-cysteine or NAC influx to cells. The latter effect was not demonstrable in vivo in the DU145 xenograft.
Significance to metallomicsCopper is an essential element required by all cells. Its metabolism is dysregulated in many diseases. Cancer cells, especially in prostate cancer, typically accumulate more copper than normal cells. In this work we explored how copper is delivered to and retained in cancer cells. We demonstrate that L-cysteine and N-acetyl-cysteine supplementation increases cellular copper retention in prostate cancer cells and in some other cell lines but amino acids in serum are not required to deliver copper ions to transporters for import into the cells tested. |
Introduction
Due to the indispensable roles of copper in biological processes and the toxicity of copper excess,1 its trafficking is normally tightly controlled. Dysregulation leads to, or results from, disorders of copper deficiency (Menkes disease)2 or overload (Wilson's disease).3 Copper imbalance is also implicated in other pathologies such as neurodegeneration4 and tumour development.5 Investigating copper transport is important for understanding its role in disease aetiology and for designing related diagnostic and therapeutic strategies.In eukaryotes, the main route of cellular copper uptake across the plasma membrane is via copper transporter 1 (CTR1, in human cells hCTR1),6 though some cell types utilise alternative mechanisms.7 Upon entry, copper ions are transported to their subcellular destinations (e.g., to copper-dependent enzymes) by specific metallochaperones,8 such as antioxidant 1 copper chaperone (ATOX1). These transfer copper directly from one binding site to another without release.9
CTR1-mediated transport of copper occurs via transmetallation involving coordinating methionine triads in the transmembrane region of CTR1, which form a selectivity filter.10,11 While copper is mainly present in plasma as copper(II), CTR1 transports Cu(I) ions.6,10,12–14 Several ferric reductases were shown to also possess cupric reductase activity,15–17 while studies on CTR1 model peptides suggested that reduction of Cu(II) bound to CTR1 could also be mediated by physiological reducing agents such as ascorbate.18,19 Other mechanistic details, such as the identity of the direct donors of copper ions from circulation to CTR1 and whether these donors are involved in the redox of copper ions during CTR1 transport, remain speculative.20 Most copper in human serum is bound to proteins.21In vitro studies have shown that cultured cells can receive copper from all the main protein carriers: ceruloplasmin, transcuprein and albumin.13,22,23 Direct copper transfer from human serum albumin (HSA) to a model hCTR1 peptide has been demonstrated spectroscopically.24
A small percentage of serum copper pool is bound to other biomolecules not as extensively studied as ceruloplasmin, transcuprein or albumin. This pool, isolated by gel filtration,25–27 ultrafiltration28,29 or dialysis,1 includes species identified as small proteins (<30 kDa) and low molecular weight (LMW) components whose abundance (1–10%) and whose upper molecular weight cut-off vary depending on the analytical method used. The functions of these species in copper transport are largely unknown, except for a 2 kDa molecule playing a role in urinary copper excretion.30 The LMW fraction probably includes copper–amino acid complexes, as first suggested several decades ago based on indirect methods including in vitro reconstitution experiments31 and thin layer chromatography, which detected copper–histidine and -threonine species.1 Computer simulations based on complex formation constants demonstrated the prevalence of copper–histidine, copper–histidine–glutamine and copper–histidine–threonine complexes in plasma.32 Other work taking into account redox equilibria suggested a copper–cysteine complex as the major LMW constituent.33 There is no consensus regarding the existence or composition of the copper-binding amino-acid pool in human serum.
An alternative strategy to illuminate the roles of amino acids in copper transport and cellular delivery is to investigate whether their presence in the extracellular medium affects copper accumulation in cells. In rat liver slices, addition of a mixture of amino acids,34 or histidine alone,35 markedly increased copper uptake, while in erythrocytes1 and cultured rat hepatocytes36 histidine alleviated the inhibitory effect of serum proteins on copper uptake. In lymphoid cells, by contrast, added histidine inhibited copper accumulation in the presence of albumin.1 Copper–histidine complex was shown to donate copper to mouse embryonic fibroblasts,13 with uptake rates comparable to those from copper–transcuprein and -albumin complexes. Investigators of the dietary interplay of amino acids and copper found that copper–amino acid complexes had higher permeability in intestinal cells in vitro compared to ionic copper,37 though in vivo studies were inconclusive.38,39
There have been no systematic studies of the effects of individual amino acids other than histidine on copper uptake into tumour cells. Such studies are particularly timely due to the emerging roles of copper in tumour signalling pathways,5 which underpin on-going clinical trials of copper chelation therapies in cancer40 and the use of copper-based radionuclide imaging of prostate tumours.41 Mechanisms of copper delivery from serum donors to copper membrane transporters (mainly hCTR1) are essentially unknown.
Against this background, we aimed to test the hypothesis that LMW components of the copper serum pool could affect copper uptake in cells by acting as copper donors to the cellular copper transporters. Adopting a radiotracer approach, we measured in vitro64Cu accumulation in prostate cancer cells (DU145) in the presence of individual amino acids selected based either on existing evidence1 of their complexation with copper in serum (L-histidine, L-threonine) or presence of chemical groups capable of copper ion coordination (L-methionine, L-cysteine). We investigated in vitro whether amino acids can affect 64Cu delivery to cells, and if so, by what mechanism? We then investigated whether the conclusions drawn were relevant to whole-body copper trafficking to tumours in vivo using Positron Emission Tomography (PET) with 64Cu.
Methods
64Cu production
[64Cu]CuCl2 was produced as previously described42 by proton-irradiating a solid 64Ni target, dissolving it in concentrated hydrochloric acid and fractionating the solution by ion-exchange chromatography. Fractions with highest 64Cu concentrations containing [64Cu]CuCl2 (specific activity 1313 ± 553 MBq μg−1 in 0.1–1.0 M hydrochloric acid) were evaporated to dryness under a N2 stream. For in vitro studies, dry 64Cu was re-dissolved in 0.9% NaCl to give a final pH of 3–5. For in vivo studies, dry 64Cu was re-dissolved in 39–51 mM sodium acetate (229873, Sigma), giving a final pH of 6–7.Cell culture
Prostate cancer DU145 (HTB-81), PC3 (CRL-1435) and breast cancer MDA-MB-231 (HTB-26) cells were purchased from ATCC (Middlesex, UK). Melanoma A375, ovarian cancer SKOV-3 and IGROV-1 cells were generously shared by Prof Sophia Karagiannis (King's College London). Cells were cultured in RPMI 1640 medium (R0883, Sigma) with 10% (v/v) fetal bovine serum (FBS), 2 mM L-glutamine and penicillin–streptomycin, except MDA-MB-231 cells which were cultured in low-glucose Dulbecco's Modified Eagle Medium (DMEM, D5546, Sigma), supplemented as above. All cells were kept in a humidified incubator at 37 °C and 5% CO2.In vitro cellular accumulation of 64Cu
Cells grown as monolayer in 24-well plates were washed twice with PBS and incubated with 10 kBq [64Cu]CuCl2 per well at 37 °C under 5% CO2. Three or four technical replicates were used for each condition. For the amino acid solutions prepared in Hank's Balanced Salt Solution (HBSS) no significant pH drift was observed, as monitored by the benchtop pH meter (SevenCompact S220, Mettler Toledo, Fig. S1, ESI†). The pH of the final incubation medium after addition of amino acids and [64Cu]CuCl2 measured using indicator papers (Whatman, 2613-991) was close to 7 in all cases. Following incubation, cells were washed twice with phosphate-buffered saline (PBS, Sigma, 806552) and lysed using NaOH (0.1 M). Radioactivity associated with cell lysates or extracellular medium/PBS washes was gamma counted (Wallac gamma counter). Percentage cellular accumulation of 64Cu was converted to a ratio of the intracellular/extracellular concentrations of 64Cu; for some purposes these values were normalised to ratios obtained in control conditions (e.g., in HBSS or in serum without added amino acids) and expressed as fold change. Results are depicted as means ± SD of independent biological experiments (performed on different days). Amino acids were purchased as follows: L-cysteine hydrochloride (093727) and D-cysteine hydrochloride (036386) from Fluorochem, N-acetyl-L-cysteine (A7250) and L-cystine (PHR1323) from Sigma and L-threonine (138930050) from Acros Organics. Detailed descriptions of the protocols and data analysis are in the ESI.†In vitro cellular efflux of 64Cu
DU145 cells, prepared as described above, were pre-incubated (30 minutes) with 64Cu and L-Cys (100 μM) in HBSS (vs. control: 64Cu in HBSS only). Radioactive HBSS was replaced with non-radioactive HBSS and cells were incubated for 1 hour (efflux step). 64Cu was measured in the pellet and in the replaced HBSS.Cell fractionation
Cell fractionation was performed after incubating DU145 cells with 10 kBq [64Cu]CuCl2 in 1% FBS in HBSS buffer for 90 minutes. Abcam's cell fractionation kit (109719) was used to prepare highly-enriched cytoplasmic, mitochondrial and nuclear fractions. Following incubation with [64Cu]CuCl2 and PBS washes as described above, cells were harvested with trypsin. Subsequent steps followed the fractionation kit protocol, except that a replacement buffer A, free of ethylenediaminetetraacetic acid (EDTA), was used. Subcellular fractions were collected and gamma counted. Purity of cytoplasmic and mitochondrial fractions was shown by western blotting (Fig. S6A; see protocols in the ESI†).Instant thin layer chromatography (iTLC)
100 mm × 10 mm strips of glass fibre paper impregnated with silica gel (SG, Agilent Technologies A120B12) were developed in a 50 mL plastic Falcon tube with 700 μL of iTLC mobile phase (detailed below). Radioactivity was visualised by electronic autoradiography with the Cyclone Plus Storage Phosphor Scanner (PerkinElmer, C431200) with Cyclone Plus 5.0 software. Method 1-Assessing the oxidation state of 64Cu: the mobile phase was 100 μM bicinchoninic acid (BCA, Sigma D8234) in water, giving retention factor (Rf) = 0 for 64Cu(II) and Rf = 1 for 64Cu(I); for method validation see Fig. S3 (ESI†). Method 2-Distinguishing free 64Cu from L-cysteine-complexed 64Cu: the mobile phase was butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid
acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; Rf = 1 for free 64Cu and Rf = 0.7 for 64Cu–L-cysteine complex.
4; Rf = 1 for free 64Cu and Rf = 0.7 for 64Cu–L-cysteine complex.
Size-exclusion high-performance liquid chromatography (HPLC)
L-Cysteine solutions in HBSS (150 μL) were incubated with 1–1.5 MBq [64Cu]CuCl2 (15 μL) for 20 minutes at room temperature (RT) and analysed on an Agilent Technologies 1200 Series HPLC unit with a BioSep™ 5 μm SEC-s2000 145 Å column (300 × 7.8 mm), coupled with a UV/vis detector at 254 nm and a LabLogic Flow-Count radioactivity detector with a sodium iodide probe (B-FC-3200). The mobile phase was 0.9% (w/v) NaCl at a flow rate of 0.8 mL min−1.Human serum preparation
Blood collection from consenting healthy volunteers was approved by a local ethics committee. Blood samples were harvested in serum-separating tubes containing clotting activator (Medisave, 367958), incubated at RT for 30 minutes and centrifuged at 22 °C, 1500 relative centrifugal force (RCF) for 10 minutes. Serum was separated, filtered through a 0.45 μm filter and stored at 4 °C for use within 72 h, or at −20 °C for later use.Measuring thiol concentration using Ellman's reagent
HBSS buffer containing L-cysteine and [64Cu]CuCl2 was added to wells of two 24-well plates-one with and one without DU145 cells. Plates were incubated for 1 hour at 37 °C in the humidified incubator. HBSS was sampled (20 μL) before and after the incubation period and added in triplicate to a 96-well plate, followed by 200 μL of the 0.2 mM Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid), DTNB, Sigma D8130) in phosphate buffer (0.1 M, pH = 8.0, 5 mM EDTA). Plates were incubated for 5 minutes at RT and monitored at 412 nm using a SpectroStar Nano plate reader. The unknown thiol concentrations were derived from a linear regression using L-cysteine standards ranging from 2.44 to 1.25 mM.Measuring total glutathione (GSH) by an enzymatic recycling method in cells treated with buthionine sulfoximine (BSO)
DU145 cells were grown for 24 hours in 24-well plates to 80% confluency, in unmodified complete growth medium either with or without 1 mM BSO (Focus Biomolecules, 10-4572). The following day, cells were incubated in HBSS supplemented with 1% (v/v) FBS and 10 kBq [64Cu]CuCl2 (1 hour, with/without 100 μM L-cysteine), then harvested and counted with the haemocytometer, using Trypan blue to confirm viability. In vitro cellular accumulation of 64Cu was assessed by gamma counting. Total GSH content in the cell extracts was then measured by an enzymatic-recycling method43 from a linear regression using GSH standards ranging from 0.4125 to 26.4 nM. Calculated GSH content was normalised to the cell count and nominal cell volume44 to yield intracellular GSH concentration. Glutathione reductase (G3664), β-NADPH (N7505) and DTNB (D8130) were from Sigma.DU145 xenograft model
Animal experiments were performed in accordance with the Animals (Scientific Procedures) Act, 1986 using protocols approved by the Animal Welfare and Ethical Review Body for King's College London (St Thomas’ Campus). To generate the prostate cancer xenograft model, 8 week old male SCID/beige mice (Envigo, UK) were injected subcutaneously in the left shoulder with 4 × 106 DU145 cells in PBS (100 μL). Mice were regularly monitored for health status and imaged when tumours reached 4–8 mm diameter (3–4 weeks).PET/CT imaging and ex vivo biodistribution
All administered substances were prepared <24 hours before injection and passed through a 0.22 μM filter. NAC was dissolved in saline and neutralised to pH 6–7 with 3 M NaOH, [64Cu]Cu–acetate was prepared as described as above. Animals were anaesthetised with isoflurane (2.0–3.0% in O2, flow rate 1 L min−1) and injected via the tail vein with either 150 mg kg−1 NAC (n = 3) or saline (n = 3) over the course of 2 minutes. After 5 minutes all animals were i.v. injected with 4 ± 2 MBq [64Cu]Cu–acetate and immediately imaged by PET for 1 hour, followed by a CT scan, using a nanoScan® PET/CT scanner (Mediso Medical Imaging Systems, Budapest, Hungary). Images were acquired and reconstructed using Nucline software (version 1.02, Mediso Ltd, Budapest, Hungary) and analysed using VivoQuant software (Version 3.5). Mice were sacrificed 75 min post-injection (p.i.) using cervical dislocation and their tissues were harvested, weighed and gamma counted (Wallac gamma counter).Statistical analysis
Statistical analysis was done with GraphPad Prism 8. Data were first assessed for normal and lognormal distribution using Shapiro–Wilk test. For in vitro64Cu accumulation, paired analysis was performed to match the amino acid supplemented groups with their control groups, to account for the inter-experimental variability of 64Cu accumulation (Fig. S2, ESI†). To compare 2 groups a paired ratio t-test was used. In the experiments with >2 groups, data points were transformed to log values and analysed by one-way Anova with Dunnett's post hoc test for multiple comparisons with the HBSS control or with Sidak's correction for multiple comparisons of pre-selected columns. For longitudinal in vitro64Cu accumulation results, a two-way Anova was used with Dunnett's (for >2 groups) or Bonferroni's (2 groups) post hoc test. To compare 3 groups not following normal and lognormal distribution a rank-based paired Friedman test was used with Dunn's correction. In the in vivo experiment, 64Cu uptake over 1 hour was calculated as an area under the curve (AUC). AUC values were then compared between the NAC pre-injection and control groups using a two-tailed unpaired t-test. p values below 0.05 were considered significant.Results
L-Cysteine-induced increase in 64Cu accumulation in cancer cell lines
The effect of individual amino acids on 64Cu accumulation in vitro was initially measured in HBSS, since media such as DMEM already contain a mixture of amino acids. Among L-histidine, L-methionine, L-threonine and L-cysteine, only L-cysteine had a statistically significant effect on 64Cu accumulation (p = 0.001) in DU145 prostate cancer cells (Fig. 1A). After 90 minutes of incubation, the intracellular/extracellular concentration ratio of 64Cu reached 195 ± 212 in HBSS only, while with addition of 100 μM L-Cys it reached 829 ± 634. The high variability in accumulation of 64Cu on different experimental days (Fig. S2A and B, ESI†) prompted us to pair results obtained in HBSS and with amino acids within each independent experiment. This analysis revealed that, on average, addition of L-cysteine to HBSS increased the 64Cu intracellular/extracellular ratios by 5.15 ± 2.98 fold (p = 0.001). | ||
| Fig. 1 The effect of added amino acids on 64Cu accumulation in DU145 cells. The effect of L-cysteine on 64Cu accumulation in additional cancer cell lines is shown in Fig. S1D (ESI†). (A) 64Cu accumulation (90 min) in DU145 cells in HBSS (control) with added L-histidine (L-His), L-methionine (L-Met), L-cysteine (L-Cys) and L-threonine (L-Thr), all at 100 μM concentration, n = 3. (B) 64Cu accumulation (60 min) in DU145 cells in HBSS (control) with added (10 μM) L-Cys or L-cystine (L-Cys2), n = 3. Int./ext. ratios of 64Cu conc. were analysed by one-way Anova with Dunnett's post hoc test for multiple comparisons with the HBSS control (A) or by a ratio paired t-test (B). Graphs represent mean ± SD of control-normalised values. p < 0.05 is shown as *, p < 0.01 as **. | ||
To check for effects of oxidation of L-cysteine to its disulfide L-cystine in HBBS (considering the presence of oxidising copper ions), we compared the effects of the L-cystine and L-cysteine on 64Cu accumulation. 10 μM concentration was selected due to poor L-cystine solubility. Only the reduced form increased the intracellular/extracellular 64Cu ratios (p = 0.03, Fig. 1B). 64Cu was present in HBSS at picomolar concentration and we confirmed with Ellman's reagent that under those conditions L-cysteine was not oxidised to L-cystine (Fig. S2C, ESI†).
Preliminary tests for this L-cysteine-specific effect were also performed in five other cancer cell lines. In prostate cancer PC3 and ovarian cancer SK-OV-3 cells, L-cysteine (100 μM) increased the intracellular/extracellular 64Cu ratios but had no measurable effect in ovarian cancer IGROV-1, melanoma A375 or breast cancer MDA-MB-231 cells (Fig. S2D, ESI†). Since the effect was most pronounced in DU145 cells, these were selected as an experimental system to investigate its mechanisms.
64Cu complexation with L-cysteine does not enhance 64Cu accumulation in cells
With the aim of determining the role of the amino acid-bound copper species in cellular copper accumulation, we tested whether L-cysteine could significantly complex 64Cu under the experimental conditions used for 64Cu accumulation studies. Complexation of 64Cu with increasing L-cysteine concentration in HBSS was monitored by HPLC (Fig. 2A) and iTLC (Fig. 2B). In the HPLC system used, unchelated 64Cu does not elute and remains bound to the stationary phase. With the addition of 1 mM, but not 100 μM L-cysteine, 64Cu eluted from the column, proving that 64Cu was complexed more strongly by L-cysteine than by the stationary phase only at the higher cysteine concentration. In the iTLC method, free 64Cu in HBSS migrated to the solvent front (Rf = 1) and its behaviour was unchanged by the addition of 10 or 100 μM L-cysteine, but 1 mM L-cysteine changed the Rf to 0.7, again suggesting significant complexation of 64Cu by L-cysteine only at high concentrations.We then tested the effect of different L-cysteine concentrations on cellular 64Cu accumulation. The presence of 10 or 100 μM L-cysteine (concentrations under which chromatography suggested that 64Cu is only very weakly bound by L-cysteine) increased the intracellular/extracellular concentration ratio of 64Cu after incubating cells with 64Cu for at least 30 minutes (Fig. 2C). This suggests that 64Cu–L-cysteine complexes did not contribute to the enhanced cellular 64Cu accumulation. On the contrary, complexation might lead to decreased accumulation, since L-cysteine at 1 mM (a concentration at which the chromatography experiments showed strong copper complexation) had an inhibitory effect on 64Cu accumulation at 7 (p < 0.0001) and 15 minutes (p = 0.002).
L-Cysteine reduces 64Cu(II) to 64Cu(I) in HBSS but reduction does not underlie the effect on 64Cu accumulation
To test the hypothesis that the enhancement of 64Cu accumulation by L-cysteine is attributable to 64Cu(II) reduction, we repeated the above 64Cu cellular accumulation experiment in the presence of other known 64Cu(II)-reducing agents D-cysteine, glutathione (GSH) and ascorbate. The capacity of all of these agents, including L-cysteine, to reduce 64Cu(II) was confirmed by iTLC (Fig. S3B, ESI†), but only L-cysteine was able to increase 64Cu accumulation in DU145 cells (p = 0.03, Fig. 3). | ||
| Fig. 3 The effect of reducing agents on 64Cu accumulation in DU145 cells. Conditions used: L-Cys and D-cysteine (D-Cys) – 100 μM, 90 min, ascorbate – 1 mM, 90 min, glutathione (GSH) – 10 μM, 60 min, n = 3. 64Cu(II) to 64Cu(I) reduction by all reductants was demonstrated by ITLC SG method, shown in Fig. S3B (ESI†). All experiments were done in HBSS and normalised to HBSS control. A more detailed version of the figure is shown in Fig. S4 (ESI†). Graph represents mean ± SD of control-normalised values. Int./ext. ratios of 64Cu conc. were analysed by a two-way Anova with Sidak's post hoc test for multiple comparisons with the HBSS control. p < 0.05 is shown as *. | ||
Cellular 64Cu accumulation enhancement by L-cysteine is due to intracellular events following cellular uptake of L-cysteine
The hypotheses tested above (64Cu complexation or reduction by L-cysteine) focused on plausible extracellular mechanisms by which L-cysteine might modulate 64Cu accumulation in HBSS. After rejecting these hypotheses, we evaluated an alternative explanation: that L-cysteine affects 64Cu retention by an intracellular rather than extracellular process. This concept was tested by pre-incubating DU145 cells with L-cysteine, followed by washing before adding 64Cu (Fig. 4A). These experiments showed that pre-incubation of cells with L-cysteine enhanced subsequent 64Cu accumulation (Fig. 4B). Moreover, the higher the L-cysteine concentration in HBSS during the pre-incubation step, the greater the subsequent increase in 64Cu accumulation. Importantly, the effect could be partially blocked by the addition, during L-cysteine incubation, of an excess (1 mM) of L-serine and L-threonine, which are known to block L-cysteine uptake45 (Fig. 4C). The L-cysteine effect is absent if cells are incubated in DMEM, which could be explained by the presence of competing amino acids (Fig. S5, ESI†). In the subsequent experiment (Fig. 4D), we introduced a second wash-out step following L-cysteine pre-incubation and removal. After a very short (1 minute) wash-out period, L-cysteine increased the intracellular/extracellular 64Cu ratio 3.00 ± 1.24 times (p < 0.001). Longer wash-out periods reduced the enhancement to a non-significant level to 1.51 ± 0.14-fold by 30 minutes (p = 0.1), to 1.30 ± 0.04-fold by 90 minutes (p = 0.47) and by 180 minutes of wash-out there was no enhancement. These results further support the hypothesis that the L-cysteine effect depends on its cellular accumulation.L-Cysteine increases 64Cu cellular retention mainly in the cytoplasm but independently from GSH synthesis
Next, we examined the possible ways in which increased intracellular L-cysteine could influence 64Cu trafficking. To determine whether L-cysteine promotes 64Cu delivery into cells or promotes its cellular retention, we measured 64Cu efflux from DU145 cells after loading with 64Cu, with and without 100 μM L-cysteine. Preloading cells with L-cysteine resulted in 4-fold decrease in % 64Cu efflux compared to cells loaded with 64Cu without L-cysteine (p = 0.03, Fig. 5A). This suggests that L-cysteine inhibits efflux of 64Cu rather than promoting its uptake.We then addressed the intracellular localisation of 64Cu using a commercial cell fractionation kit employing different detergents to isolate cytoplasm, mitochondria and nuclei (Fig. S6A, ESI†). To circumvent potential problems caused by copper chelation, the kit was modified to exclude EDTA (which did not change the observed 64Cu fractionation – Fig. S6B, ESI†). Cells accumulating 64Cu in the presence and absence of L-cysteine had an almost identical pattern of 64Cu distribution among the compartments: 85–86% in cytoplasm, 10–11% in mitochondria and 2–3% in nuclei (Fig. 5B).
A proposed cytoplasmic copper binding partner is GSH46 and L-cysteine is a substrate for its synthesis.47 We therefore tested the role of GSH synthesis in the L-cysteine effect on 64Cu retention. We first showed that incubating DU145 cells with 100 μM L-cysteine for 1 hour (the time-scale on which L-cysteine affects 64Cu accumulation) did not significantly change intracellular GSH levels (measured by an enzymatic recycling method as 6.79 ± 2.94 mM in control cells and 6.41 ± 2.61 mM in L-cysteine-treated cells (Fig. 5C), p = 0.68). Depletion of GSH in DU145 cells by BSO treatment (Fig. 5C) did not affect basal 64Cu accumulation in HBSS (p = 0.95), nor did it affect 64Cu accumulation enhancement caused by L-cysteine (Fig. 5D). Preliminary experiments exploring the molecular identity of intracellular 64Cu (Fig. S7A, ESI†) by size-exclusion chromatography showed that the most (87.90 ± 1.18%) was tightly bound to proteins; this percentage was similar (84.62 ± 2.37%) when cells were co-incubated with L-cysteine. Thus, GSH did not directly participate in the enhancement of 64Cu retention by L-cysteine.
N-Acetylcysteine (NAC) affects 64Cu similarly to L-cysteine and both retain their effect in the presence of serum
So far, we used HBSS buffer to delineate the effect of incubating cells with individual amino acids. However, HBSS composition is far from physiological conditions under which copper enters human cells in serum. Therefore, some of the previous experiments were replicated in the presence of 10% FBS, which is commonly used in tissue culture. Under these conditions (Fig. 6A) our preliminary experiments showed that the effect of L-cysteine addition was even more pronounced than before (the intracellular/extracellular 64Cu ratio increased 9.71 ± 0.73 fold) and D-cysteine also exhibited an effect, albeit smaller (increasing the int./ext. 64Cu ratio by 4.23 ± 0.45 fold). Notably, in the presence of 10% FBS (Fig. 6B) and in 100% human (Fig. 6C) or mouse serum (Fig. 6D), co-incubation with high concentrations of L-cysteine (1 mM) also enhanced 64Cu accumulation over a 1 hour period (contrary to what was found for serum-free experiments where such high concentrations inhibited 64Cu accumulation). Addition of NAC (100 μM and 1 mM), an approved drug in humans which acts as a L-cysteine precursor in cells,48 significantly increased 64Cu accumulation in 10% FBS (4.24 ± 1.58 fold at 1 mM, p = 0.006, Fig. 6B), and in mouse serum (2.75 ± 0.55 fold at 1 mM, p = 0.03, Fig. 6D). The persistence of these effects in serum suggested that they could be physiologically relevant, which warranted progression to in vivo studies.The effect of N-acetylcysteine on in vivo trafficking of 64Cu in a prostate cancer mouse model
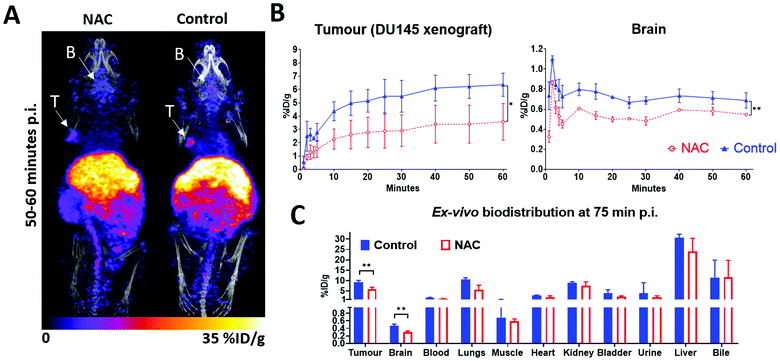
The enhancement of 64Cu accumulation in cancer cells by added thiols encouraged us to explore the use of in vivo NAC supplementation as a way to improve intratumoural accumulation of 64Cu (which has been used clinically, as [64Cu]CuCl2 for prostate cancer imaging).41 NAC was chosen for the in vivo work since it is approved for use in humans and its pharmacokinetics has been studied in mice.49,50 Prior work has shown that it has a more pronounced effect than L-cysteine on the replenishment of the intracellular L-cysteine levels51 and in our hands (Fig. 6D) it enhanced cellular 64Cu accumulation in mouse serum more than L-cysteine did. The dose and form of NAC administration (i.v. bolus injection, 150 mg kg−1) were chosen based on a previous report49 that at 10 minutes p.i. it yielded plasma NAC concentrations approaching 1 mM. The timing of NAC administration 5 minutes before 64Cu-acetate injection was guided by the in vitro wash-out data (Fig. 4D). Mice bearing DU145 xenografts were injected with NAC or saline (control), followed by 64Cu acetate 5 minutes later. PET images revealed 64Cu-acetate biodistribution in agreement with previous reports,52 exhibiting pronounced liver uptake and biliary excretion (Fig. 7A). As expected, there was pronounced accumulation of 64Cu in DU145 tumours, which however was reduced, not enhanced, by NAC supplementation (p < 0.05). At 50–60 minutes p.i. the mean uptake was 6.36 ± 0.87%ID per g in the control group and 3.58 ± 1.38%ID per g in the NAC group. NAC treatment also significantly (p < 0.01) decreased brain 64Cu uptake, particularly before 30 minutes p.i. Ex vivo biodistribution analysis at 75 min p.i. confirmed that 64Cu uptake values (%ID per g) in tumour and brain were both reduced in the NAC group (p < 0.01, Fig. 7C).Discussion
L-Cysteine substantially increased 64Cu accumulation in DU145 prostate cancer cells, whereas L-histidine, L-methionine and L-threonine did not (Fig. 1). L-Cysteine had a similar effect in PC3 (prostate cancer) and in SK-OV-3 (ovarian cancer) cells (Fig. S2D, ESI†). The effect is not universal as it was not seen in IGROV-1 (ovarian cancer), MDA-MB-231 (breast cancer) and A375 (melanoma) cells. The mechanism of this phenomenon was investigated in more depth using DU145 cells (the main findings are reviewed in Scheme 1).Potentially, complexation of copper by L-cysteine could facilitate copper delivery to CTR1, or participate in an additional transport system.53 However, the experiments suggested that the enhancement of 64Cu accumulation in DU145 cells was not due to complexation of copper by L-cysteine in the extracellular medium, because the presence of L-cysteine at levels high enough to significantly complex copper inhibited rather than enhanced 64Cu accumulation (Fig. 2).
Another possible explanation was based on the premise that CTR1 transports reduced Cu(I) ions,10 which is consistent with reports that in vitro uptake of 64Cu is augmented in the presence of reducing agents.6,12,13,54 To determine whether reduction of Cu(II) by L-cysteine could be the cause of 64Cu accumulation enhancement, we tested a range of alternative reducing agents reported (and proven by our experiments) to be capable of reducing Cu(II). Amongst them, only L-cysteine had an effect on 64Cu accumulation (Fig. 3). These results do not refute the well-documented specificity of CTR1 for Cu(I) – it is possible that tracer amounts of 64Cu used in our experimental system (0.6 nM) were already effectively reduced on the surface of DU145 cells, and did not require added reducing agents. They do, however, suggest that reduction to Cu(I) is not the key factor in enhancement of 64Cu accumulation by L-cysteine.
Subsequent experiments suggested that L-cysteine, rather than inducing changes in extracellular 64Cu speciation, mediates changes in the intracellular 64Cu trafficking or efflux. This was first suggested by the observed delay between L-cysteine addition and its first detectable effect on 64Cu only after 30 minutes of incubation (Fig. 2), and confirmed by observing that 64Cu accumulation was enhanced by pre-incubating cells with L-cysteine, followed by its removal from the HBSS before addition of 64Cu (Fig. 4); hence L-cysteine did not need to be present in the HBSS to exert its effect. Addition of L-serine (1 mM) and L-threonine (1 mM) during the pre-incubation with L-cysteine partially blocked the L-cysteine (100 μM) effect. An earlier report showed that in erythrocytes, these amino acids supplied at 2.5 mM concentration blocked L-cysteine uptake by 95%.45 The effect of L-cysteine was also diminished if the pre-incubation step was followed by a wash-out period. These results together suggest that L-cysteine is first taken up by DU145 cells and then exerts its effect on 64Cu trafficking intracellularly. L-Cysteine supply to cells mainly occurs in the form of L-cystine via the xCT transporter.55 However, since L-cystine supplementation did not influence 64Cu accumulation in DU145 cells (Fig. 1B), we conclude that the transport of (reduced) L-cysteine itself was responsible for the downstream effect. Our experiments do not unambiguously identify which L-cysteine transport systems are operative in DU145 cells, but the blocking effect of L-serine and L-threonine suggests the involvement of the ASCT (Alanine, Serine, Cysteine Transporter) system.56–58 Further support for the hypothesis that L-cysteine exerts its effect from within the cell comes from the observation that efflux of previously accumulated 64Cu from cells was markedly slowed by prior incubation with L-cysteine (Fig. 5).
Fractionation of DU145 cells following 64Cu incubation with and without L-cysteine showed that the vast majority of 64Cu accumulating in cells was present in the cytoplasm. It should be noted that we used a fractionation kit designed for protein analysis so we cannot exclude the possibility that upon cell lysis and fractionation, some of the 64Cu was exchanged between its usual binding partners or moved to different organelles – though we did remove copper chelating agents from the buffers to minimise this possibility.
We hypothesised that GSH might mediate the observed effect because L-cysteine availability is typically rate-limiting for GSH synthesis.55 GSH is known to bind Cu(I)59 and may participate in intracellular copper trafficking.46,60–64 Our results, however, did not support this hypothesis, at least in DU145 cells: BSO treatment drastically depleted GSH levels but did not change basal 64Cu accumulation or the magnitude of the L-cysteine effect on 64Cu accumulation (Fig. 5). Moreover, L-cysteine incubation did not enhance intracellular GSH levels.
The intracellular events underlying the effect of L-cysteine and N-acetyl-cysteine on 64Cu retention remain unclear. The fast onset of the effect precludes the involvement of protein synthesis in the process. Since the intracellular copper ions are bound with attomolar affinities,65 it is unlikely that copper–L-cysteine complexes with their much lower stability constants66 would be able to account for intracellular buffering of copper. In another line of explanation, we suggest that L-cysteine exerted an indirect effect on 64Cu trafficking, which increased 64Cu flux to the retention sites. Potential ways in which this could occur are outlined below.
(1) L-Cysteine could affect the kinetics of copper transfer between the intracellular chaperones. Small molecules, such as glutamate,67 glutathione or L-cysteine68 have been shown to modulate the kinetics of copper transfer between its binding partners.
(2) An increase in the intracellular L-cysteine levels could influence the redox-sensitive residues of proteins or LMW ligands. This could change the availability of copper-binding sites or modulate protein function.69 Intracellular redox status regulates ATOX1-mediated copper trafficking and efflux from cells.70,71 ATOX1 redox status is dependent on the intracellular oxidised/reduced glutathione ratio but not on the total glutathione levels.70 This warrants further investigation to test whether 64Cu accumulation enhancement is mediated by the effect of L-cysteine supplementation on oxidised/reduced glutathione ratios.
(3) Potentially, the observed effect of L-cysteine might involve a secondary effect of L-cysteine metabolism. Apart from contributing to glutathione synthesis, L-cysteine can undergo numerous intracellular catabolic pathways, such as generation of taurine, pyruvate or hydrogen sulfide.72 Hydrogen sulfide can trap 64Cu at very low levels73 or regulate copper export systems in Mycobacterium tuberculosis74 and in human neuroblastoma cells.75S-Adenosylhomocysteine hydrolase, one of the key enzymes in the metabolism of thiol-containing amino acids, was found to bind appreciable amounts of copper in mouse livers76 and its expression is regulated by copper availability in mice.77
To what extent is modulation of cellular copper accumulation by L-cysteine important in vivo? The finding that results obtained in HBSS were replicated in the presence of human or mouse serum (Fig. 6), suggests that L-cysteine still affects accumulation of 64Cu bound to some physiological serum carriers. Moreover, the effect persists in the presence of competing non-radioactive copper and L-cysteine present in serum. Reported L-cysteine levels in human serum are in the range 10–34 μM78,79 and vary in disease states such as Alzheimer's disease,80 coronary heart disease81 and cancer.82 Cancer cells often show high demand for L-cysteine, or indeed L-cysteine dependency.83 Both types of ASCT transporters are upregulated in various human cancers, including prostate cancer.84,85
To test whether the effect of L-cysteine, or its “prodrug” form NAC could enhance 64Cu accumulation in prostate cancer in vivo, we used PET imaging in a DU145 subcutaneous xenograft mouse model. However, i.v. injection of 150 mg kg−1 NAC 5 minutes before 64Cu–acetate injection slightly decreased, rather than increased, 64Cu accumulation in DU145 xenografts. This could be due to metabolism of NAC prior to reaching the DU145 tumour; a change in the DU145 cell phenotype in a xenograft compared to cultured DU145 cells (such as transporter expression); the effect of the other cell types and extracellular matrix at the tumour site; or differences in the 64Cu speciation between serum (used in the in vitro experiments) and whole blood.
Unexpectedly, NAC treatment had an inhibitory effect on brain 64Cu accumulation at all time-points between 0–60 minutes p.i., but most prominently between 4–20 minutes p.i. This was confirmed by both the PET image and by ex vivo biodistribution. Whether NAC is capable of crossing the blood–brain barrier (BBB) is unclear: studies with 14C-50,86 or 13C-labelled49 NAC in mice were inconclusive but there is some evidence for NAC modulating brain redox status.49 NAC could also affect 64Cu speciation in serum, regional blood flow or post-transcriptional regulation of copper transporters.
Conclusions
We have shown that delivery of ionic copper to cellular transporters on prostate cancer DU145 cells can occur in vitro without the involvement of LMW intermediates (complexes of L-histidine, L-cysteine, L-threonine or L-methionine). This warrants further investigation of the roles of individual copper-transporting proteins as copper donors, or the ternary complexes of proteins with copper and LMW species.87 Notwithstanding, in some cell lines we also found previously unreported effects on 64Cu accumulation by thiols (L-cysteine and NAC), not due to extracellular speciation changes but via intracellular mechanisms that are independent of GSH synthesis. Further investigation in vivo of the link between thiols and copper metabolism in the context of tumour biology could help elucidate the mechanism of increased copper retention in cancers. Additionally, gaining insight into the suppression of 64Cu brain uptake by NAC supplementation may enhance understanding of dysregulated redox states and copper metabolism in the brain, and suggest strategies to modulate pathological brain copper accumulation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Professor Wolfgang Maret for reviewing this manuscript and providing invaluable advice. This work was supported by the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by EPSRC Programme Grant [#EP/S032789/1]. King's College London and UCL Comprehensive Cancer Imaging Centre is funded by the CRUK and EPSRC in association with the MRC and DoH (England). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the DoH. JJB is supported by UK Medical Research Council (MRC) [MR/N013700/1], Guy's and St Thomas’ Charity and King’s College London member of the MRC Doctoral Training Partnership in Biomedical Sciences. FAS was supported by the CRUK City of London Centre Award [C7893/A26233]. GF was funded by the King's College London and Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1).Notes and references
- M. C. Linder, Biochemistry of Copper, Springer, US, Boston, 1991 Search PubMed.
- J. H. Menkes, Menkes disease and Wilson disease: two sides of the same copper coin Part 1: Menkes disease, Eur. J. Paediatr. Neurol., 1999, 3, 147–158 Search PubMed.
- A. Czlonkowska, T. Litwin, P. Dusek, P. Ferenci, S. Lutsenko, V. Medici, J. K. Rybakowski, K. H. Weiss and M. L. Schilsky, Wilson disease, Nat. Rev. Dis. Primers, 2018, 4, 21 Search PubMed.
- D. J. Waggoner, T. B. Bartnikas and J. D. Gitlin, The Role of Copper in Neurodegenerative Disease, Neurobiol. Dis., 1999, 6, 221–230 Search PubMed.
- D. Denoyer, S. Masaldan, S. La Fontaine and M. A. Cater, Targeting copper in cancer therapy: ‘Copper That Cancer’, Metallomics, 2015, 7, 1459–1476 Search PubMed.
- J. Lee, M. M. Pena, Y. Nose and D. J. Thiele, Biochemical characterization of the human copper transporter Ctr1, J. Biol. Chem., 2002, 277, 4380–4387 Search PubMed.
- A. M. Zimnicka, K. Ivy and J. H. Kaplan, Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake, Am. J. Physiol.: Cell Physiol., 2011, 300, C588–C599 Search PubMed.
- H. Ohrvik, J. Aaseth and N. Horn, Orchestration of dynamic copper navigation – new and missing pieces, Metallomics, 2017, 9, 1204–1229 Search PubMed.
- T. D. Rae, P. J. Schmidt, R. A. Pufahl, V. C. Culotta and T. V. O’Halloran, Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase, Science, 1999, 284, 805–808 Search PubMed.
- F. Ren, B. L. Logeman, X. Zhang, Y. Liu, D. J. Thiele and P. Yuan, X-ray structures of the high-affinity copper transporter Ctr1, Nat. Commun., 2019, 10, 1386 Search PubMed.
- E. B. Maryon, S. A. Molloy, K. Ivy, H. Yu and J. H. Kaplan, Rate and regulation of copper transport by human copper transporter 1 (hCTR1), J. Biol. Chem., 2013, 288, 18035–18046 Search PubMed.
- L. Jiang, Y. Tu, X. Hu, A. Bao, H. Chen, X. Ma, T. Doyle, H. Shi and Z. Cheng, Pilot Study of (64)Cu(I) for PET Imaging of Melanoma, Sci. Rep., 2017, 7, 2574 Search PubMed.
- T. Z. Kidane, R. Farhad, K. J. Lee, A. Santos, E. Russo and M. C. Linder, Uptake of copper from plasma proteins in cells where expression of CTR1 has been modulated, BioMetals, 2012, 25, 697–709 Search PubMed.
- S. Schwab, J. Shearer, S. E. Conklin, B. Alies and K. L. Haas, Sequence proximity between Cu(II) and Cu(I) binding sites of human copper transporter 1 model peptides defines reactivity with ascorbate and O2, J. Inorg. Biochem., 2016, 158, 70–76 Search PubMed.
- S. Wyman, R. J. Simpson, A. T. McKie and P. A. Sharp, Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro, FEBS Lett., 2008, 582, 1901–1906 Search PubMed.
- R. S. Ohgami, D. R. Campagna, A. McDonald and M. D. Fleming, The Steap proteins are metalloreductases, Blood, 2006, 108, 1388–1394 Search PubMed.
- G. H. Gauss, M. D. Kleven, A. K. Sendamarai, M. D. Fleming and C. M. Lawrence, The Crystal Structure of Six-transmembrane Epithelial Antigen of the Prostate 4 (Steap4), a Ferri/Cuprireductase, Suggests a Novel Interdomain Flavin-binding Site, J. Biol. Chem., 2013, 288, 20668–20682 Search PubMed.
- K. L. Haas, A. B. Putterman, D. R. White, D. J. Thiele and K. J. Franz, Model Peptides Provide New Insights into the Role of Histidine Residues as Potential Ligands in Human Cellular Copper Acquisition via Ctr1, J. Am. Chem. Soc., 2011, 133, 4427–4437 Search PubMed.
- M. J. Pushie, K. Shaw, K. J. Franz, J. Shearer and K. L. Haas, Model Peptide Studies Reveal a Mixed Histidine-Methionine Cu(I) Binding Site at the N-Terminus of Human Copper Transporter 1, Inorg. Chem., 2015, 54, 8544–8551 Search PubMed.
- J. H. Kaplan and E. B. Maryon, How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal, Biophys. J., 2016, 110, 7–13 Search PubMed.
- M. C. Linder, Ceruloplasmin and other copper binding components of blood plasma and their functions: an update, Metallomics, 2016, 8, 887–905 Search PubMed.
- M. Moriya, Y. H. Ho, A. Grana, L. Nguyen, A. Alvarez, R. Jamil, M. L. Ackland, A. Michalczyk, P. Hamer, D. Ramos, S. Kim, J. F. Mercer and M. C. Linder, Copper is taken up efficiently from albumin and alpha2-macroglobulin by cultured human cells by more than one mechanism, Am. J. Physiol.: Cell Physiol., 2008, 295, C708–C721 Search PubMed.
- D. T. Gordon, A. S. Leinart and R. J. Cousins, Portal copper transport in rats by albumin, Am. J. Physiol., 1987, 252, E327–E333 Search PubMed.
- E. Stefaniak, D. Plonka, S. C. Drew, K. Bossak-Ahmad, K. L. Haas, M. J. Pushie, P. Faller, N. E. Wezynfeld and W. Bal, The N-terminal 14-mer model peptide of human Ctr1 can collect Cu(II) from albumin. Implications for copper uptake by Ctr1, Metallomics, 2018, 10, 1723–1727 Search PubMed.
- P. L. Wirth and M. C. Linder, Distribution of copper among components of human serum, J. Natl. Cancer Inst., 1985, 75, 277–284 Search PubMed.
- K. C. Weiss and M. C. Linder, Copper transport in rats involving a new plasma protein, Am. J. Physiol., 1985, 249, E77–E88 Search PubMed.
- A. Montaser, C. Tetreault and M. Linder, Comparison of Copper Binding Components in Dog Serum with Those in Other Species, Proc. Soc. Exp. Biol. Med., 1992, 200, 321–329 Search PubMed.
- S. Catalani, M. Paganelli, M. E. Gilberti, L. Rozzini, F. Lanfranchi, A. Padovani and P. Apostoli, Free copper in serum: An analytical challenge and its possible applications, J. Trace Elem. Med. Biol., 2018, 45, 176–180 Search PubMed.
- G. A. McMillin, J. J. Travis and J. W. Hunt, Direct Measurement of Free Copper in Serum or Plasma Ultrafiltrate, Am. J. Clin. Pathol., 2009, 131, 160–165 Search PubMed.
- L. W. Gray, F. Peng, S. A. Molloy, V. S. Pendyala, A. Muchenditsi, O. Muzik, J. Lee, J. H. Kaplan and S. Lutsenko, Urinary copper elevation in a mouse model of Wilson's disease is a regulated process to specifically decrease the hepatic copper load, PLoS One, 2012, 7, e38327 Search PubMed.
- P. Z. Neumann and A. Sass-Kortsak, The state of copper in human serum: evidence for an amino acid-bound fraction, J. Clin. Invest., 1967, 46, 646–658 Search PubMed.
- V. Brumas, N. Alliey and G. Berthon, A new investigation of copper(II)-serine, copper(II)-histidine-serine, copper(II)-asparagine, and copper(II)-histidine-asparagine equilibria under physiological conditions, and implications for simulation models relative to blood plasma, J. Inorg. Biochem., 1993, 52, 287–296 Search PubMed.
- L. C. Tran-Ho, P. M. May and G. T. Hefter, Complexation of copper(I) by thioamino acids. Implications for copper speciation in blood plasma, J. Inorg. Biochem., 1997, 68, 225–231 Search PubMed.
- D. I. Harris and A. Sass-Kortsak, The influence of amino acids on copper uptake by rat liver slices, J. Clin. Invest., 1967, 46, 659–667 Search PubMed.
- P. Z. Neumann and M. Silverberg, Active Copper Transport in Mammalian Tissues—a Possible Role in Wilson's Disease, Nature, 1966, 210, 414–416 Search PubMed.
- H. M. Darwish, J. C. Cheney, R. C. Schmitt and M. J. Ettinger, Mobilization of copper(II) from plasma components and mechanisms of hepatic copper transport, Am. J. Physiol., 1984, 246, G72–G79 Search PubMed.
- S. Gao, T. Yin, B. Xu, Y. Ma and M. Hu, Amino acid facilitates absorption of copper in the Caco-2 cell culture model, Life Sci., 2014, 109, 50–56 Search PubMed.
- J. Bertinato, C. Lavergne, N. A. Vu, L. J. Plouffe, C. Wood, P. Griffin and C.-W. Xiao, L-Lysine supplementation does not affect the bioavailability of copper or iron in rats, J. Trace Elem. Med. Biol., 2016, 38, 194–200 Search PubMed.
- G. V. Mitchell and M. Y. Jenkins, Effect of Excess L-Lysine on Rat Growth and on Plasma and Tissue Concentrations of Copper, Iron and Zinc, J. Nutr. Sci. Vitaminol., 1983, 29, 709–715 Search PubMed.
- S. Baldari, G. Di Rocco and G. Toietta, Current Biomedical Use of Copper Chelation Therapy, Int. J. Mol. Sci., 2020, 21, 1069 Search PubMed.
- A. Piccardo, F. Paparo, M. Puntoni, S. Righi, G. Bottoni, L. Bacigalupo, S. Zanardi, A. DeCensi, G. Ferrarazzo, M. Gambaro, F. G. Ruggieri, F. Campodonico, L. Tomasello, L. Timossi, S. Sola, E. Lopci and M. Cabria, (64)CuCl2 PET/CT in Prostate Cancer Relapse, J. Nucl. Med., 2018, 59, 444–451 Search PubMed.
- M. S. Cooper, M. T. Ma, K. Sunassee, K. P. Shaw, J. D. Williams, R. L. Paul, P. S. Donnelly and P. J. Blower, Comparison of (64)Cu-complexing bifunctional chelators for radioimmunoconjugation: labeling efficiency, specific activity, and in vitro/in vivo stability, Bioconjugate Chem., 2012, 23, 1029–1039 Search PubMed.
- I. Rahman, A. Kode and S. K. Biswas, Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method, Nat. Protoc., 2006, 1, 3159–3165 Search PubMed.
- C. L. Kuksin D, Analyzing NCI-60 Cancer Cell Lines, https://www.nexcelom.com/training-and-support/white-papers/accurately-measure-cell-size-of-nci-60-cancer-cell-lines/, accessed 20.04.2020.
- T. Ishii, Y. Sugita and S. Bannai, Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine, J. Cell. Physiol., 1987, 133, 330–336 Search PubMed.
- E. B. Maryon, S. A. Molloy and J. H. Kaplan, Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1, Am. J. Physiol.: Cell Physiol., 2013, 304, C768–C779 Search PubMed.
- S. C. Lu, Glutathione synthesis, Biochim. Biophys. Acta, 2013, 1830, 3143–3153 Search PubMed.
- G. Tardiolo, P. Bramanti and E. Mazzon, Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases, Molecules, 2018, 23, 3305 Search PubMed.
- J. Zhou, L. D. Coles, R. V. Kartha, N. Nash, U. Mishra, T. C. Lund and J. C. Cloyd, Intravenous Administration of Stable-Labeled N-Acetylcysteine Demonstrates an Indirect Mechanism for Boosting Glutathione and Improving Redox Status, J. Pharm. Sci., 2015, 104, 2619–2626 Search PubMed.
- L. I. McLellan, A. D. Lewis, D. J. Hall, J. D. Ansell and C. R. Wolf, Uptake and distribution of N-acetylcysteine in mice: tissue-specific effects on glutathione concentrations, Carcinogenesis, 1995, 16, 2099–2106 Search PubMed.
- X. He, X. Wu, W. Shi and H. Ma, Comparison of N-acetylcysteine and cysteine in their ability to replenish intracellular cysteine by a specific fluorescent probe, Chem. Commun., 2016, 52, 9410–9413 Search PubMed.
- J. B. Torres, E. M. Andreozzi, J. T. Dunn, M. Siddique, I. Szanda, D. R. Howlett, K. Sunassee and P. J. Blower, PET Imaging of Copper Trafficking in a Mouse Model of Alzheimer Disease, J. Nucl. Med., 2016, 57, 109–114 Search PubMed.
- M. J. Walsh, S. D. Goodnow, G. E. Vezeau, L. V. Richter and B. A. Ahner, Cysteine Enhances Bioavailability of Copper to Marine Phytoplankton, Environ. Sci. Technol., 2015, 49, 12145–12152 Search PubMed.
- S. S. Percival and E. D. Harris, Ascorbate enhances copper transport from ceruloplasmin into human K562 cells, J. Nutr., 1989, 119, 779–784 Search PubMed.
- S. C. Lu, Regulation of glutathione synthesis, Mol. Aspects Med., 2009, 30, 42–59 Search PubMed.
- Y. Kanai and M. A. Hediger, The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects, Pflügers Archiv, 2004, 447, 469–479 Search PubMed.
- J. L. Arriza, M. P. Kavanaugh, W. A. Fairman, Y. N. Wu, G. H. Murdoch, R. A. North and S. G. Amara, Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family, J. Biol. Chem., 1993, 268, 15329–15332 Search PubMed.
- N. Utsunomiya-Tate, H. Endou and Y. Kanai, Cloning and Functional Characterization of a System ASC-like Na + -dependent Neutral Amino Acid Transporter, J. Biol. Chem., 1996, 271, 14883–14890 Search PubMed.
- M. T. Morgan, L. A. H. Nguyen, H. L. Hancock and C. J. Fahrni, Glutathione limits aquacopper(I) to sub-femtomolar concentrations through cooperative assembly of a tetranuclear cluster, J. Biol. Chem., 2017, 292, 21558–21567 Search PubMed.
- K. K. Tong and H. J. McArdle, Copper uptake by cultured trophoblast cells isolated from human term placenta, Biochim. Biophys. Acta, 1995, 1269, 233–236 Search PubMed.
- H. H. Chen, I. S. Song, A. Hossain, M. K. Choi, Y. Yamane, Z. D. Liang, J. Lu, L. Y. Wu, Z. H. Siddik, L. W. Klomp, N. Savaraj and M. T. Kuo, Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1, Mol. Pharmacol., 2008, 74, 697–704 Search PubMed.
- N. V. Dolgova, C. Yu, J. P. Cvitkovic, M. Hodak, K. H. Nienaber, K. L. Summers, J. J. H. Cotelesage, J. Bernholc, G. A. Kaminski, I. J. Pickering, G. N. George and O. Y. Dmitriev, Binding of Copper and Cisplatin to Atox1 Is Mediated by Glutathione through the Formation of Metal–Sulfur Clusters, Biochemistry, 2017, 56, 3129–3141 Search PubMed.
- W. C. J. Singleton, K. T. McInnes, M. A. Cater, W. R. Winnall, R. McKirdy, Y. Yu, P. E. Taylor, B.-X. Ke, D. R. Richardson, J. F. B. Mercer and S. La Fontaine, Role of Glutaredoxin1 and Glutathione in Regulating the Activity of the Copper-transporting P-type ATPases, ATP7A and ATP7B, J. Biol. Chem., 2010, 285, 27111–27121 Search PubMed.
- J. H. Freedman, M. R. Ciriolo and J. Peisach, The role of glutathione in copper metabolism and toxicity, J. Biol. Chem., 1989, 264, 5598–5605 Search PubMed.
- M. T. Morgan, D. Bourassa, S. Harankhedkar, A. M. McCallum, S. A. Zlatic, J. S. Calvo, G. Meloni, V. Faundez and C. J. Fahrni, Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 12167 Search PubMed.
- A. Rigo, A. Corazza, M. L. di Paolo, M. Rossetto, R. Ugolini and M. Scarpa, Interaction of copper with cysteine: stability of cuprous complexes and catalytic role of cupric ions in anaerobic thiol oxidation, J. Inorg. Biochem., 2004, 98, 1495–1501 Search PubMed.
- E. Stefaniak and W. Bal, Cu(II) Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function, Inorg. Chem., 2019, 58, 13561–13577 Search PubMed.
- A. Santoro, N. Ewa Wezynfeld, M. Vašák, W. Bal and P. Faller, Cysteine and glutathione trigger the Cu–Zn swap between Cu(II)-amyloid-β4-16 peptide and Zn7-metallothionein-3, Chem. Commun., 2017, 53, 11634–11637 Search PubMed.
- L. B. Poole, The basics of thiols and cysteines in redox biology and chemistry, Free Radical Biol. Med., 2015, 80, 148–157 Search PubMed.
- Y. Hatori, S. Clasen, N. M. Hasan, A. N. Barry and S. Lutsenko, Functional partnership of the copper export machinery and glutathione balance in human cells, J. Biol. Chem., 2012, 287, 26678–26687 Search PubMed.
- Y. Hatori, Y. Yan, K. Schmidt, E. Furukawa, N. M. Hasan, N. Yang, C.-N. Liu, S. Sockanathan and S. Lutsenko, Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway, Nat. Commun., 2016, 7, 10640 Search PubMed.
- M. H. Stipanuk, J. E. Dominy, Jr., J.-I. Lee and R. M. Coloso, Mammalian Cysteine Metabolism: New Insights into Regulation of Cysteine Metabolism, J. Nutr., 2006, 136, 1652S–1659S Search PubMed.
- S. Sarkar, Y. S. Ha, N. Soni, G. I. An, W. Lee, M. H. Kim, P. T. Huynh, H. Ahn, N. Bhatt, Y. J. Lee, J. Y. Kim, K. M. Park, I. Ishii, S.-G. Kang and J. Yoo, Immobilization of the Gas Signaling Molecule H2S by Radioisotopes: Detection, Quantification, and In Vivo Imaging, Angew. Chem., Int. Ed., 2016, 55, 9365–9370 Search PubMed.
- V. Saini, K. C. Chinta, V. P. Reddy, J. N. Glasgow, A. Stein, D. A. Lamprecht, M. A. Rahman, J. S. Mackenzie, B. E. Truebody, J. H. Adamson, T. T. R. Kunota, S. M. Bailey, D. R. Moellering, J. R. Lancaster and A. J. C. Steyn, Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis, Nat. Commun., 2020, 11, 557 Search PubMed.
- N. Goto, H. Hara, M. Kondo, N. Yasuda, T. Kamiya, K. Okuda and T. Adachi, Hydrogen sulfide increases copper-dependent neurotoxicity via intracellular copper accumulation, Metallomics, 2020, 868–875 Search PubMed.
- K. E. Bethin, N. Petrovic and M. J. Ettinger, Identification of a major hepatic copper binding protein as S-adenosylhomocysteine hydrolase, J. Biol. Chem., 1995, 270, 20698–20702 Search PubMed.
- K. E. Bethin, T. R. Cimato and M. J. Ettinger, Copper binding to mouse liver S-adenosylhomocysteine hydrolase and the effects of copper on its levels, J. Biol. Chem., 1995, 270, 20703–20711 Search PubMed.
- D. P. Jones, J. L. Carlson, V. C. Mody, J. Cai, M. J. Lynn and P. Sternberg, Redox state of glutathione in human plasma, Free Radical Biol. Med., 2000, 28, 625–635 Search PubMed.
- N. Psychogios, D. D. Hau, J. Peng, A. C. Guo, R. Mandal, S. Bouatra, I. Sinelnikov, R. Krishnamurthy, R. Eisner and B. Gautam, The human serum metabolome, PLoS One, 2011, 6, e16957 Search PubMed.
- M. T. Heafield, S. Fearn, G. B. Steventon, R. H. Waring, A. C. Williams and S. G. Sturman, Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease, Neurosci. Lett., 1990, 110, 216–220 Search PubMed.
- Y. Özkan, E. Özkan and B. Şimşek, Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease, Int. J. Cardiol., 2002, 82, 269–277 Search PubMed.
- F. Al-Awadi, M. Yang, Y. Tan, Q. Han, S. Li and R. M. Hoffman, Human Tumor Growth in Nude Mice Is Associated with Decreased Plasma Cysteine and Homocysteine, Anticancer Res., 2008, 28, 2541–2544 Search PubMed.
- J. A. Combs and G. M. DeNicola, The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival, Cancers, 2019, 11, 678 Search PubMed.
- D. M. Schuster, C. Nanni and S. Fanti, Evaluation of Prostate Cancer with Radiolabeled Amino Acid Analogs, J. Nucl. Med., 2016, 57, 61s–66s Search PubMed.
- Q. Wang, R. A. Hardie, A. J. Hoy, M. van Geldermalsen, D. Gao, L. Fazli, M. C. Sadowski, S. Balaban, M. Schreuder, R. Nagarajah, J. J. Wong, C. Metierre, N. Pinello, N. J. Otte, M. L. Lehman, M. Gleave, C. C. Nelson, C. G. Bailey, W. Ritchie, J. E. Rasko and J. Holst, Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development, J. Pathol., 2015, 236, 278–289 Search PubMed.
- S. A. Farr, H. F. Poon, D. Dogrukol-Ak, J. Drake, W. A. Banks, E. Eyerman, D. A. Butterfield and J. E. Morley, The antioxidants α-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice, J. Neurochem., 2003, 84, 1173–1183 Search PubMed.
- S.-J. Lau and B. Sarkar, Ternary Coordination Complex between Human Serum Albumin, Copper(II), and L-Histidine, J. Biol. Chem., 1971, 246, 5938–5943 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0mt00161a |
| This journal is © The Royal Society of Chemistry 2020 |