Distribution of aluminium in hydrated leaves of tea (Camellia sinensis) using synchrotron- and laboratory-based X-ray fluorescence microscopy†
Abstract
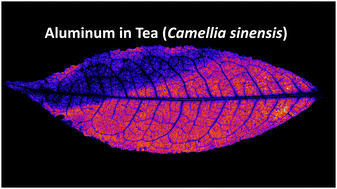
Aluminium (Al) is highly toxic to plant growth, with soluble concentrations being elevated in the ∼40% of arable soils worldwide that are acidic. Determining the distribution of Al in plant tissues is important for understanding the mechanisms by which it is toxic and how some plants tolerate high concentrations. Synchrotron- and laboratory-based X-ray fluorescence microscopy (XFM) is a powerful technique to quantitatively analyse the distribution of elements, including in hydrated and living plants. However, analysis of light elements (z < phosphorus) is extremely challenging due to signal losses in air, and the unsuitability of vacuum environments for (fresh) hydrated plant tissues. This study uses XFM in a helium environment to avoid Al signal loss to reveal the distribution of Al in hydrated plant tissues of Tea (Camellia sinensis). The results show that Al occurs in localised areas across the foliar surface, whereas in cross-sections Al is almost exclusively concentrated in the apoplastic space above and in between adaxial epidermal cells. This distribution of Al is related to the Al tolerance of this species, and accumulation of phytotoxic elements in the apoplastic space, away from sensitive processes such as photosynthesis in the palisade mesophyll cells, is a common tolerance mechanism reported in many different plant species. This study develops an XFM method on both synchrotron and laboratory sources that overcomes the drawbacks of existing analytical techniques, permitting measurement of light elements down to Al in (fresh) hydrated plant tissues.
- This article is part of the themed collection: Phytometallomics


 Please wait while we load your content...
Please wait while we load your content...