Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection†
Abstract
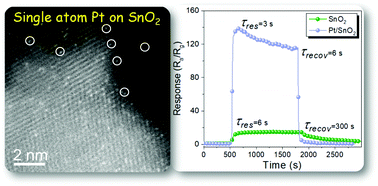
Single atom Pt functionalized SnO2 ultrathin films are synthesized by atomic layer deposition (ALD) for application as sensing layers in resistive gas sensors. Here it is shown that the electronic conductivity of the SnO2 ultrathin films is very sensitive to the exposure to triethylamine (TEA), and that the thickness of the SnO2 films (from 4 to 18 nm) has a crucial effect on the sensor response. The 9 nm thick SnO2 film shows the best response to TEA, while a further decrease in the film thickness, i.e., 4 nm, leads to a very weak response due to the two orders of magnitude lower carrier concentration. Single atom Pt catalysts deposited on the 9 nm SnO2 film result in an unexpectedly high enhancement in the sensor response and also a decrease of the sensor working temperature. Consequently, Pt/SnO2 thin film sensors show the highest response of 136.2 to 10 ppm TEA at an optimal temperature of 200 °C (that of a pristine SnO2 film sensor is 260 °C), which is improved by a factor of 9 compared to that of pristine SnO2. Moreover, the Pt/SnO2 sensor exhibits an ultrahigh sensitivity of 8.76 ppm−1 and an extremely low limit of detection (LOD) of 7 ppb, which to our best knowledge are far superior to any previous report. Very fast response and recovery times (3/6 s) are also recorded, thus making our sensor platform highly suitable for highly-demanding applications. Mechanistic investigations reveal that the outstanding sensing performances originate from the synergistic combination of the optimized film thickness comparable to the Debye length of SnO2 and the spillover activation of oxygen by single atom Pt catalysts, as well as the oxygen vacancies in the SnO2 films.
- This article is part of the themed collections: Materials Horizons 10th anniversary regional spotlight collection: China, Materials Horizons Emerging Investigators Series 2020/2021 and Materials Horizons Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...