All-weather-available, continuous steam generation based on the synergistic photo-thermal and electro-thermal conversion by MXene-based aerogels†
Abstract
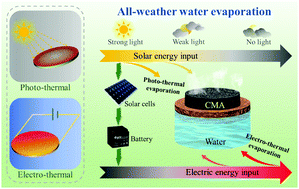
Solar energy triggered steam generation has emerged as a green and sustainable strategy that can potentially address the long-standing global freshwater scarcity issue. However, given that there is inadequate light illumination early in the morning or late afternoon or cloudy days, it is continuous steam generation in all weather that is a great challenge for the state-of-the-art solar-driven steam generators. Here, we present an all-weather-available steam generation system, which is capable of harvesting solar energy to continuously generate steam based on the alternative photo-thermal and electro-thermal conversion of crosslinked MXene aerogels (CMAs) in the day time and at night. In virtue of the strong light absorption and excellent electrical conductivity of the CMA, the constructed steam generation system can not only convert sunlight into heat for steam generation on sunny days but persistently achieve heat generation by utilization of electricity in low-light or dark circumstances. Furthermore, the ingenious introduction of solar cells-battery components makes full use of the daytime sunlight to further power the steam generation system for heating up the CMA at night, avoiding extra electrical energy input and loss. This work offers new insights into developing continuous steam generation technologies applicable to day–night alternation and complex environments.
- This article is part of the themed collections: Materials Horizons 10th anniversary regional spotlight collection: China, Horizons Community Board Collection: Solar Energy Conversion and Materials Horizons Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...