A bipolar host based high triplet energy electroplex for an over 10 000 h lifetime in pure blue phosphorescent organic light-emitting diodes†
Abstract
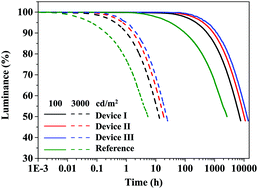
Ultimate device performances of blue phosphorescent organic light-emitting diodes, an external quantum efficiency of 27.6%, a device lifetime over 10 000 h at 100 cd m−2, and pure blue color coordinates of (0.12, 0.13), were simultaneously achieved using a device architecture employing an electroplex host. A high triplet energy electron transport type host derived from a triazine core, a carbazole substituent, and a triphenylsilyl blocking unit was newly synthesized for the purpose of developing the high emission energy electroplex host. The electroplex host functioned as a carrier balancing host for high external quantum efficiency and a lifetime extending host of pure blue phosphorescent organic light-emitting diodes. This work demonstrates ultimate device performances, high external quantum efficiency and long lifetime, in pure blue phosphorescent organic light-emitting diodes. In particular, the lifetime limit of pure blue phosphorescent organic light-emitting diodes was overcome by demonstrating an over 10 000 h lifetime.
- This article is part of the themed collections: Materials Horizons 10th anniversary regional spotlight collection: Asia-Pacific and Materials Horizons Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...