The Soret effect in dry polymer electrolyte†
Abstract
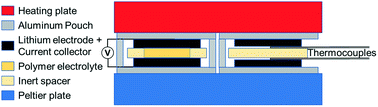
The Soret effect results in a concentration gradient when a mixture is exposed to a temperature gradient. It is a balance between diffusion of mass driven by the temperature gradient (thermal diffusion) and mass diffusion acting to remove the concentration gradient. Thus, the Soret effect is measured at steady state. In this work, the Soret effect was studied in a thermogalvanic cell with lithium metal electrodes and a dry polymer electrolyte composed of poly(ethylene oxide) and lithium bis-trifluoromethanesulfonylimide (LiTFSI). The concentration gradient was determined by measuring the voltage of the thermogalvanic cell. This was examined at several different temperature gradients and with four different salt concentrations. The Soret coefficient was found to be similar to that observed in small-molecule mixtures and electrolytes and significantly less than polymeric systems. An explanation for this unexpected result is proffered. The Soret coefficient was found to be concentration dependent, which requires further investigation. Finally, it was demonstrated that the thermogalvanic cells used to measure the Soret coefficient can also be used to generate power. Thus, polymer electrolytes are potentially of interest for waste heat recovery, and thermal diffusion might be used to improve battery efficiency.
- This article is part of the themed collection: Charge Transporting Nanostructured Polymers for Electrochemical Systems


 Please wait while we load your content...
Please wait while we load your content...
