On the intrinsic reactivity of highly potent trypanocidal cruzain inhibitors†
Abstract
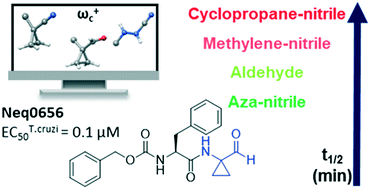
The cysteine protease cruzipain is considered to be a validated target for therapeutic intervention in the treatment of Chagas disease. Hence, peptidomimetic cruzipain inhibitors having a reactive group (known as warhead) are subject to continuous studies to discover novel antichagasic compounds. Here, we evaluated how different warheads for a set of structurally similar related compounds could inhibit the activity of cruzipain and, ultimately, their trypanocidal effect. We first investigated in silico the intrinsic reactivity of these compounds by applying the Fukui index to correlate it with the enzymatic affinity. Then, we evaluated their potency against T. cruzi (Y and Tulahuen strains), which revealed the reversible cruzain inhibitor Neq0656 as a better trypanocidal agent (ECY.strain50 = 0.1 μM; SI = 58.4) than the current drug benznidazole (ECY.strain50 = 5.1 μM; SI > 19.6). We also measured the half-life time by HPLC analysis of three lead compounds in the presence of glutathione and cysteine to experimentally assess their intrinsic reactivity. Results clearly illustrated the reactivity trend for the warheads (azanitrile > aldehyde > nitrile), where the aldehyde displayed an intermediate intrinsic reactivity. Therefore, the aldehyde bearing peptidomimetic compounds should be subject for in-depth evaluation in the drug discovery process.


 Please wait while we load your content...
Please wait while we load your content...